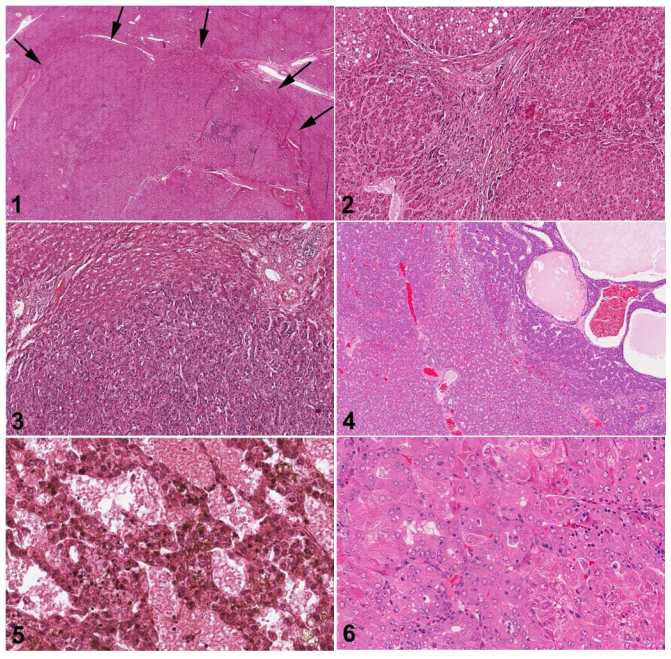
Comparative Histomorphological Review of Rat and Human Hepatocellular Proliferative Lesions
Robert Maronpot2017-01-11T22:13:34+00:00In this comparative review, histomorphological features of common nonneoplastic and neoplastic hepatocyte lesions of rats and humans are examined using H&E-stained slides. The morphological similarities and differences of both neoplastic (hepatocellular carcinoma and hepatocellular adenoma) and presumptive preneoplastic lesions (large and small cell change in humans and foci of cellular alteration in rats) [...]