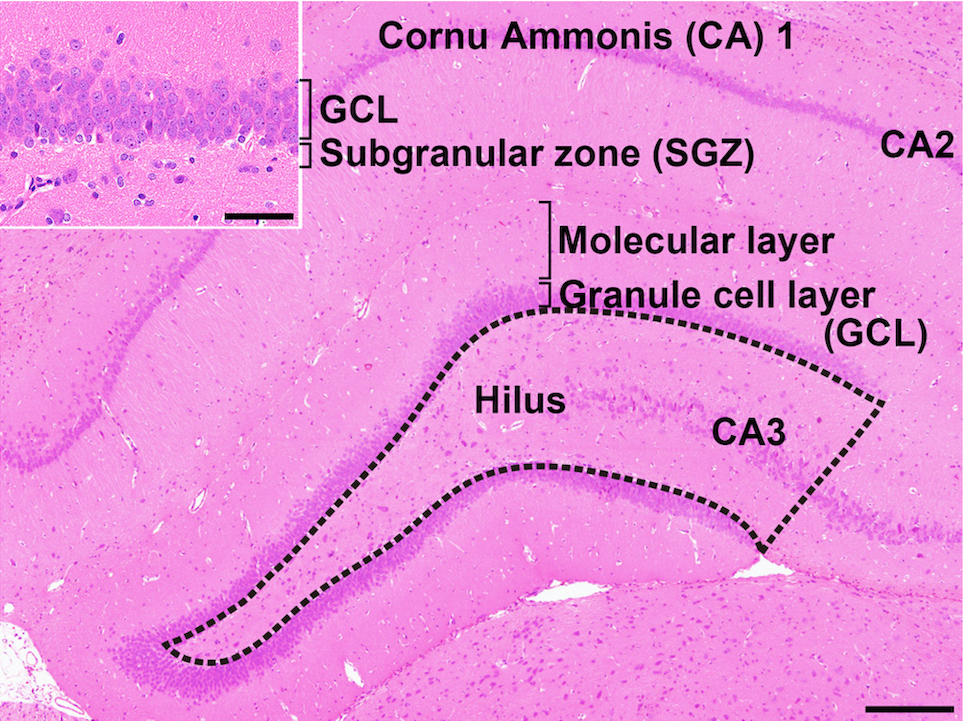
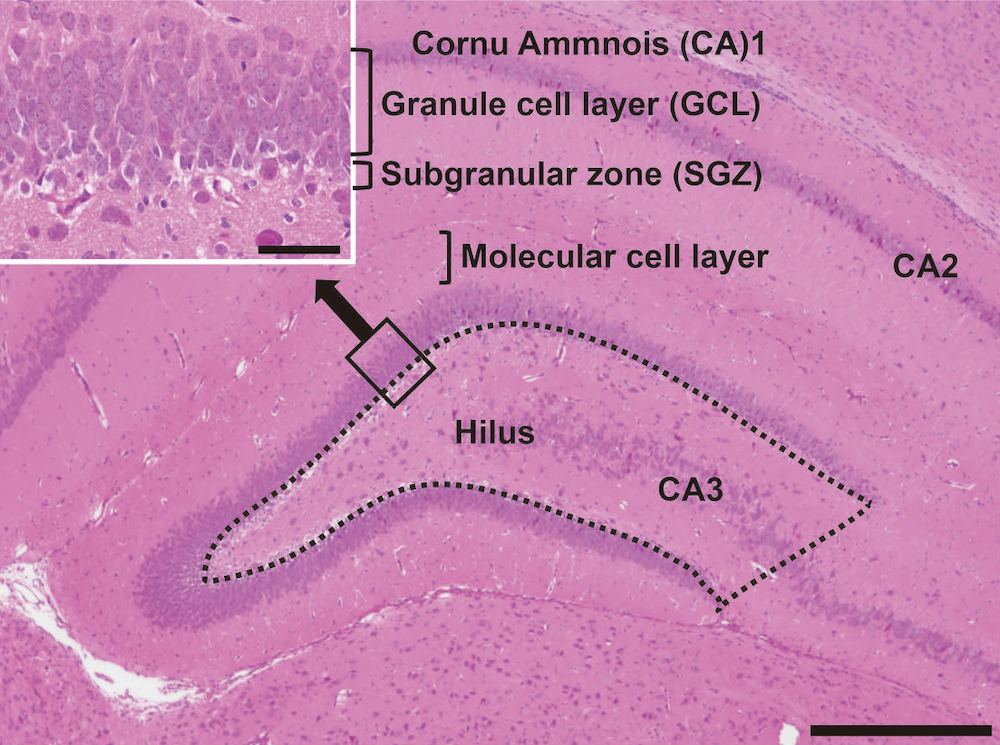
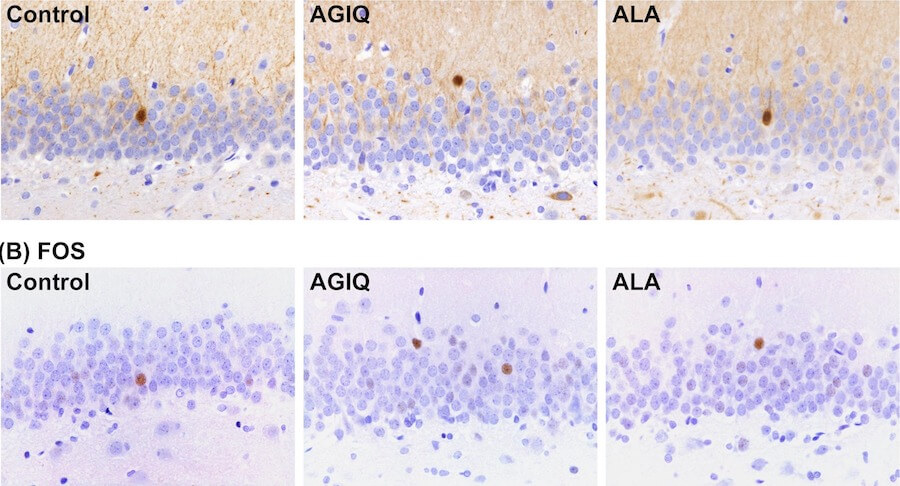
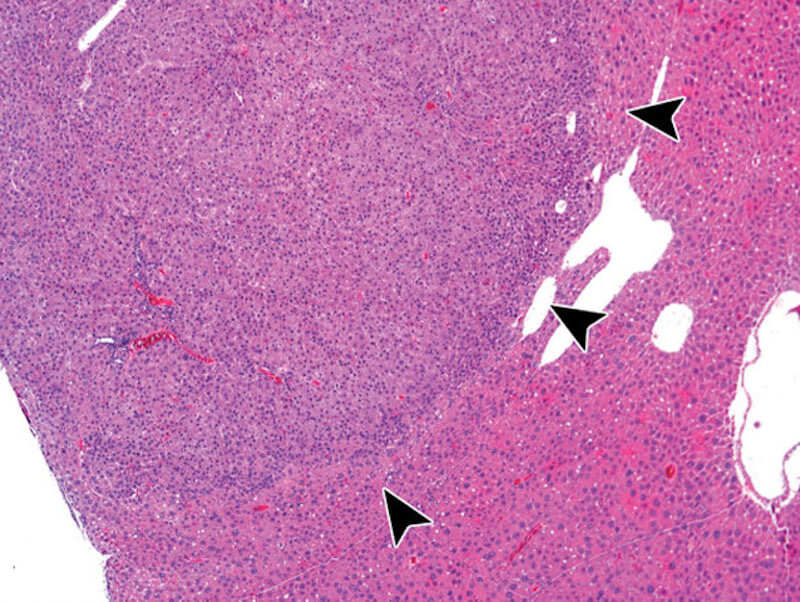
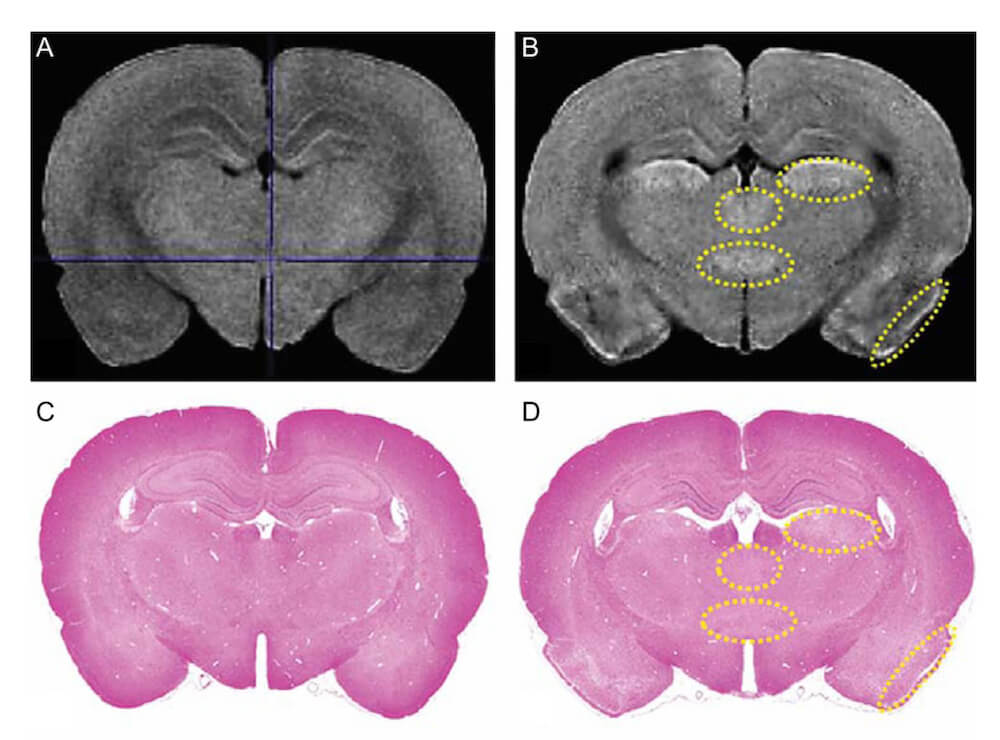
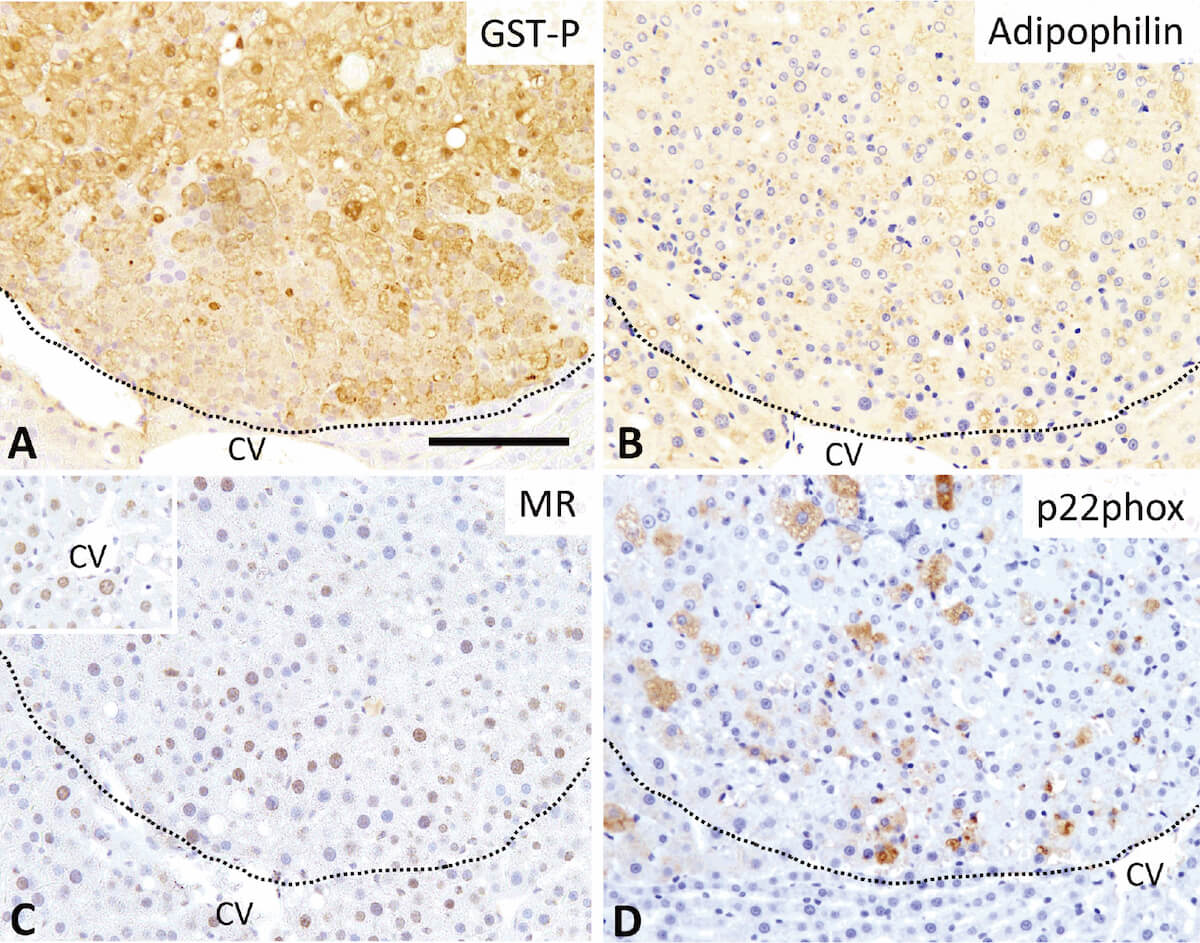
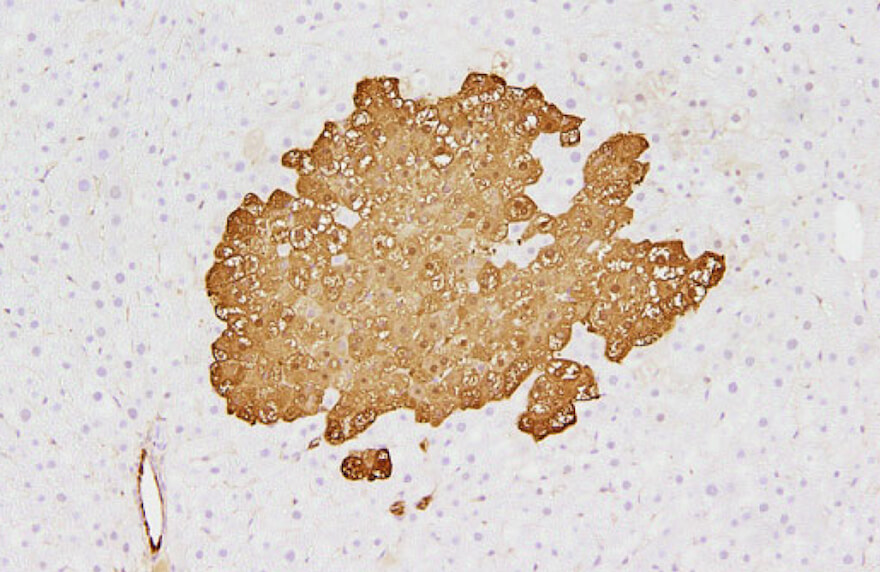
Lack of preventive effect of maternal exposure to α-glycosyl isoquercitrin and α-lipoic acid on developmental hypothyroidism-induced aberrations of hippocampal neurogenesis in rat offspring
Robert Maronpot2020-04-21T18:00:35+00:00Hypothyroidism during the developmental stage induces disruption of hippocampal neurogenesis in later life, as well as inducing oxidative stress in the brain. The present study investigated the preventive effect of co-exposure to an antioxidant on disruptive neurogenesis induced by developmental exposure to anti-thyroid agent in rats. For this purpose, we used two antioxidants, [...]