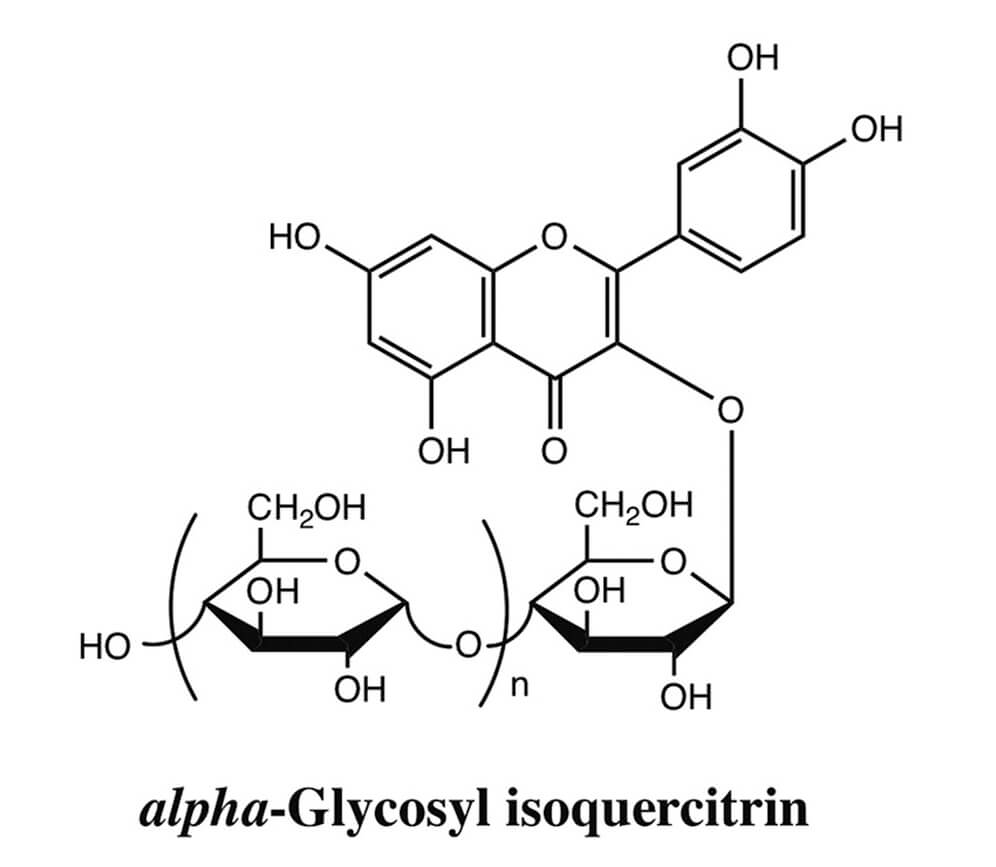
Genotoxicity evaluation of the naturally-derived food colorant, gardenia blue, and its precursor, genipin
Robert Maronpot2018-11-08T21:04:21+00:00Gardenia blue is widely used in Eastern Asia as a natural food colorant. To evaluate the genotoxic potential of gardenia blue, as well as genipin, the natural starting material from which it is produced, a GLP-compliant test battery was conducted according to OECD guidelines. No evidence of mutagenicity of gardenia blue was detected in a 5-strain bacterial reverse [...]