In this comparative review, histomorphological features of common nonneoplastic and neoplastic hepatocyte lesions of rats and humans are examined using H&E-stained slides. The morphological similarities and differences of both neoplastic (hepatocellular carcinoma and hepatocellular adenoma) and presumptive preneoplastic lesions (large and small cell change in humans and foci of cellular alteration in rats) are presented and discussed. There are major similarities in the diagnostic features, growth patterns and behavior of both rat and human hepatocellular proliferative lesions and in the process of hepatocarcinogenesis. Further study of presumptive preneoplastic lesions in humans and rats should help to further define their role in progression to hepatocellular neoplasia in both species.
Keywords
Introduction
Primary hepatocellular carcinoma (HCC) is the fifth most common cancer in the world and the third most frequent cancer-related cause of death with increasing incidence worldwide1,2,3,4. In addition, HCC is the most common primary liver malignancy in the world5,6,7. In the majority of cases, it is associated with hepatitis B or C viral infections, aflatoxicosis, and/or liver cirrhosis8,9,10,11. Other risk factors for developing HCC include alcoholic liver disease, nonalcoholic steatohepatitis, diabetes, and obesity12,13. Most patients with HCC are diagnosed at a late stage; therefore, the prognosis of HCC patients is generally very poor, with a 5-year survival rate of less than 5%7,14.
Experimental rat and mouse hepatocarcinogenesis models have been used for decades to delineate the pathogenesis of hepatic neoplasia. The rodent experimental model is used to identify potential human carcinogenic risk from exposure to drugs, environmental agents, and other xenobiotics. Rat hepatocellular adenomas (HCAs) and carcinomas are commonly used in tumor response and carcinogenicity bioassays and share some common features with human adenomas and carcinomas15.
In rat experimental models, presumptive preneoplastic foci of cellular alteration occur prior to the appearance of hepatocellular adenomas and HCC; however, there is experimental evidence that not all foci of cellular alteration progress to neoplasia and that some may actually regress16,17. Basophilic (BAS), eosinophilic (EOS), and clear cell (CLEAR) foci of cellular alteration in rats are the counterparts of human liver cell dysplasias classified as large cell change and small cell change. The detection of these presumptive preneoplastic lesions in humans may be indicative of progression towards HCC18,19,20,21 although further investigation is warranted. The purpose of this overview is to compare and contrast the morphological features of representative examples of commonly occurring human and rat hepatoproliferative lesions and to report the biology of these lesions.
Method
Paraffin blocks of adult human cases were selected from the archives of the Departments of Pathology, University Medical Center Utrecht (UMCU) and Erasmus Medical Center Rotterdam, The Netherlands. These surgical specimens were reviewed and considered unequivocal examples of human focal nodular hyperplasias (FNHs), HCCs, HCAs, large cell change (LCC) and small cell change (SCC).
Paraffin blocks of rat cases obtained from the National Toxicology Program (NTP) archives were from studies of chemical-induced liver tumors and represent diagnoses peer reviewed by experienced rodent toxicologic pathologists. Rat cases include HCCs, HCAs and basophilic, eosinophilic and clear cell foci of cellular alteration (FCAs). FNH lesions were not identified as they are rare in rats. However, this lesion was included in humans since it is one of the most common human proliferative liver lesions.
Original slides from the human and rat cases were reviewed by a medical liver pathologist and two toxicologic pathologists and selected using published diagnostic criteria8,22,23,24,25,26 to confirm original diagnoses. Once confirmed, additional sections for this study were prepared, and all hematoxylin and eosin (H&E) staining was performed simultaneously at the UMCU after collection of all unstained paraffin slides on coated glass slides (e.g., Superfrost Plus) (see Table 1).
Results
Human cases
1. Focal nodular hyperplasia (FNH)
Liver samples were derived from surgical excision (hemihepatectomy, partial liver resection or biopsy) of female patients (11/12; 92%) and one male patient (1/12; 8%) at the UMCU. The corresponding resection specimens included in the study for comparison, were grossly nodular and ranged in diameter from 2 to 17 cm. Microscopically, they had classical diagnostic features of FNH consisting of nodules composed of plates of hyperplasic hepatocytes that were two-cell layers thick and subdivided by fibrous septa (Fig. 1). Thick-walled arteries were present in the stellate scars and septa, and there were bile ductules typically located between the scars and the liver parenchyma (Fig. 2). Near the fibrous septa there were occasionally small immature cells with oval to fusiform leptochromatic nuclei and scant cytoplasm that resembled rat oval cells. In addition, transitional cells displaying characteristics of both hepatocytes and bile duct cells were also present in some samples. These results suggest the presence of “undifferentiated progenitor cells” within FNH and further suggests that the ductular reaction, at least partly, can be explained by activation of these cells27.
2. Hepatocellular carcinoma (HCC)
For the human samples, 14/16 HCC (88%) were from male patients. The morphological features consisted of a broad trabecular growth pattern of hepatocytes with occasional mixed growth patterns of trabecular/compact (Fig. 3), trabecular/acinar (Fig. 5) and sometimes a mixture of the three growth patterns. Hemorrhage, ischemic necrosis, neovascularization, angiectasis or peliosis hepatis and cystic changes were more commonly observed in these malignant tumors as compared to the other lesions evaluated.
3. Hepatocellular adenoma (HCA)
HCAs from female patients (n=15) had a maximum diameter of 16 cm. Histologically, they matched the common diagnostic criteria (see Table 2) for this benign liver neoplasm and sometimes showed focal to more diffuse steatosis, which can be observed in these tumors (Fig. 7)28,29,30.
Human cases (continued)
1. Liver cell dysplasia – large cell change (LCC) and small cell change (SCC) (human)
Large cell change: LCC (synonyms: large liver cell change (LLCC) or large liver cell dysplasia (LLCD)) has been described in detail by Anthony et al.31 and others24,32,33,34,35,36. Morphological features of hepatocytes with large cell change included cellular enlargement, nuclear pleomorphism with hyperchromasia, prominent nucleoli and occasional multinucleation. Enlargement was usually two- to three-fold and both nuclear and cytoplasmic with a normal nucleus to cytoplasmic ratio. Cytoplasmic staining was normal with occasionally more or less glycogen than that present in the surrounding liver parenchyma31. A classical example of such lesions is illustrated in Fig. 9. The selected cases of LCC (n=19) were all from 10 male patients.
Small cell change: SCC (synonyms: small liver cell change (SLCC) or small liver cell dysplasia (SLCD)) was characterized by small hepatocytes with a high nuclear:cytoplasmic (N:C) ratio. Cells were uniform and differed from cells of the surrounding parenchyma in terms of nuclear atypia and cytoplasmic staining. Fat or glycogen content sometimes differed from that in the adjacent liver parenchyma. These collections of cells with small cell change tended to produce more small round distinct foci with irregular margins similar to foci that are more closely associated with the HCCs (Fig. 11) as reported by others37,38,39. The selected SCC cases (n=17) were from 9 patients (7/9, 78% males and 2/9, 22% females). In the cases evaluated, combined areas of LCC and SCC could sometimes be observed within the same slide.
Rat cases
1. Hepatocellular carcinoma (HCC)
The HCCs reviewed, were from dosed males (3/15 HCC, 20%) and females (12/15, 80%). The morphological features were consistent with published HCC criteria26 and exhibited largely trabecular growth patterns, although mixed growth patterns (trabecular/acinar/solid) and occasionally basophilic and eosinophilic areas were present. In one case, the HCC arose within a hepatocellular adenoma and had an infiltrative growth pattern. A trabecular growth pattern with focal steatosis and mitosis is illustrated in Figure 4. As was seen in human HCCs, sometimes areas with acinar growth patterns were observed (Fig. 6). Most lesions evaluated also had hemorrhage, necrosis, pigment deposition (hemosiderin), angiectasis and/or focal fatty change.
2. Hepatocellular adenoma (HCA)
The selected hepatocellular adenomas (n=10) were from animals of both sexes (6/10, 60% females and 4/10, 40% in males). The histological features were compatible with the common diagnostic criteria (see Table 2). Sometimes, these adenomas also contained diffuse fatty change (steatosis) and angiectasis (Fig. 8).
3. Focus of cellular alteration (FCA)
Eosinophilic foci: Eosinophilic foci of cellular alteration consisted of polygonal enlarged hepatocytes with increased acidophilic staining compared with the surrounding normal liver. Clear cells (glycogen storage) were occasionally present. The cytoplasm was distinctly smooth to sometimes granular and pale pink, with a “ground-glass” appearance. Nuclei were enlarged, and nucleoli were prominent and centrally located. A classical example is illustrated in Fig. 10. For the eosinophilic foci evaluated (n=11), 10/11 (91%) were in male rats.
Basophilic foci: Basophilic foci may show some resemblance with small cell change as observed in humans. Different subtypes have been described26. The tigroid subtype consists of a focus of (small) basophilic cells distinct from the surrounding liver parenchyma and arranged in tortuous cords. Cells display large abundant basophilic bodies often arranged in clumps or long bands with a striped (“tigroid”) pattern in the paranuclear or peripheral regions of the cytoplasm (due to increased rough endoplasmic reticulum). In the samples we evaluated, the diffuse subtype was most common and consisted of small, discrete, clearly demarcated, strongly basophilic foci with irregular borders. These foci were randomly distributed within the hepatic lobule. Although different subtypes have been recognized in rodents, a typical example is shown in Fig. 12. Of the basophilic foci evaluated (n=9), 8/9 (89%) were observed in male rats.
Clear cell foci: Clear cell areas of groups of hepatocytes can be observed in HCCs in both human and rodents40,41,42,43. Clear cell foci consisted of normal or enlarged groups of cells with prominent cell membranes and distinct cytoplasmic clear spaces surrounding a densely stained centrally located nucleus. Some eosinophilic or basophilic cells were occasionally present within clear cell foci. A classical example of a clear cell focus is presented in Fig. 13.
The role of clear cell foci in hepatocarcinogenesis is elusive and poorly described, although metabolic changes in carbohydrate metabolism have been associated with HCCs in both humans and rodents41,42,44,45, and therefore these foci, as observed in the rat liver, could play a role in liver tumor formation. The selected clear cell foci (n=9) were only found in the livers of male rats.
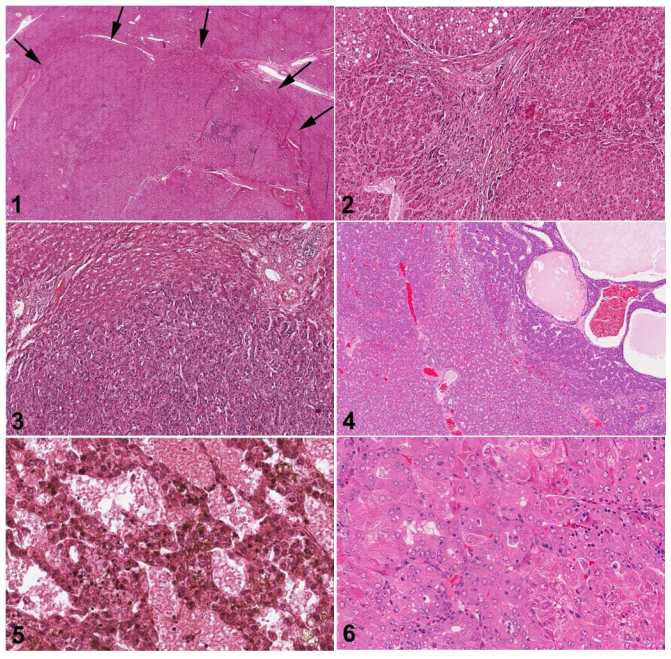
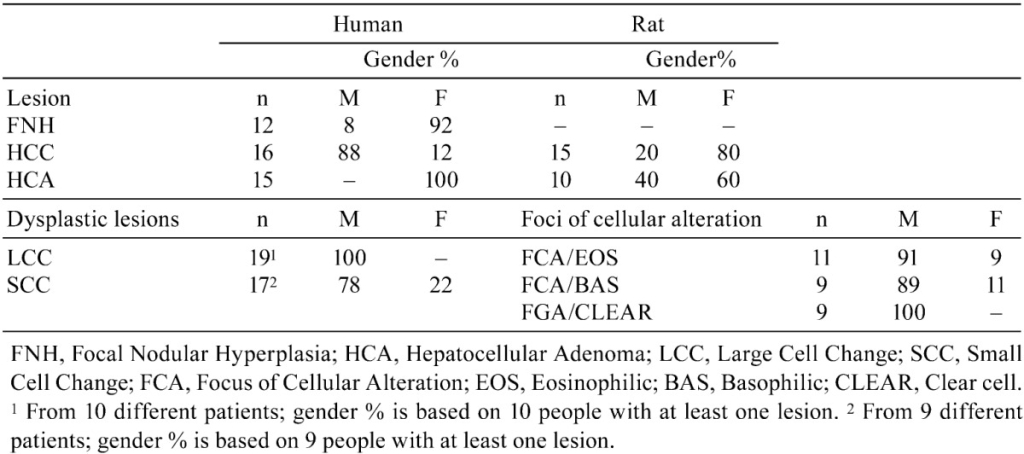
Fig. 1. Human liver. Low magnification of FNH. The upper border of the FNH is indicated by arrows with relatively normal hepatic parenchyma at the top of the figure. H&E.
Fig. 2. Human liver. Higher magnification of Fig. 1. The FNH consists of nodules composed of two-cell layers of hepatocytes subdivided by fibrous septa. Proliferative ductules are present in stellate septal scars. H&E.
Fig. 3. Human liver. Hepatocellular carcinoma composed of atypical hepatocytes arranged in a solid or trabecular growth pattern with normal hepatic parenchyma present at the top of the figure. H&E.
Fig. 4. Rat liver. Hepatocellular carcinoma with a trabecular growth pattern at the top and right of the figure. There is angiectasis in the carcinoma on the right. Normal hepatic parenchyma is present on the lower left of the figure. H&E.
Fig. 5. Human liver. High magnification of a hepatocellular carcinoma with a mixed acinar and trabecular growth pattern. H&E.
Fig. 6. Rat liver. High magnification of a hepatocellular carcinoma with a mixed acinar and trabecular growth pattern. H&E.
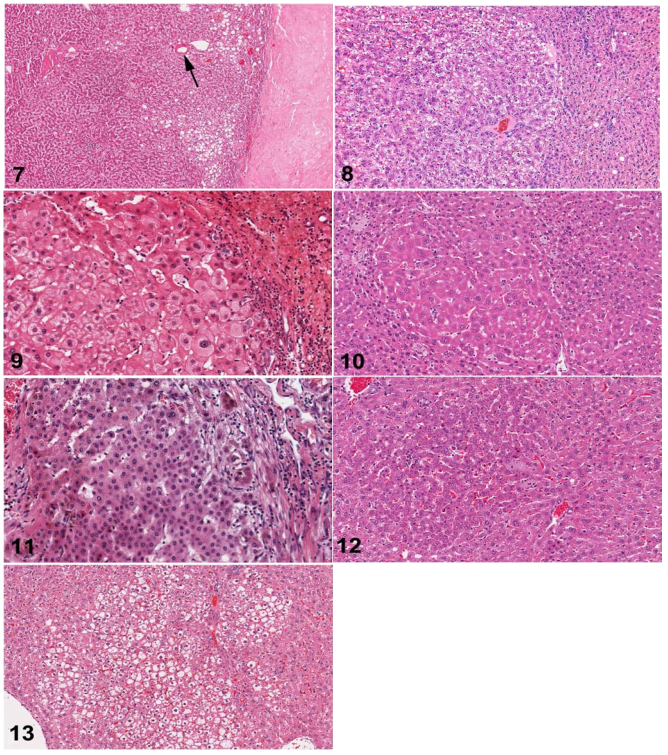
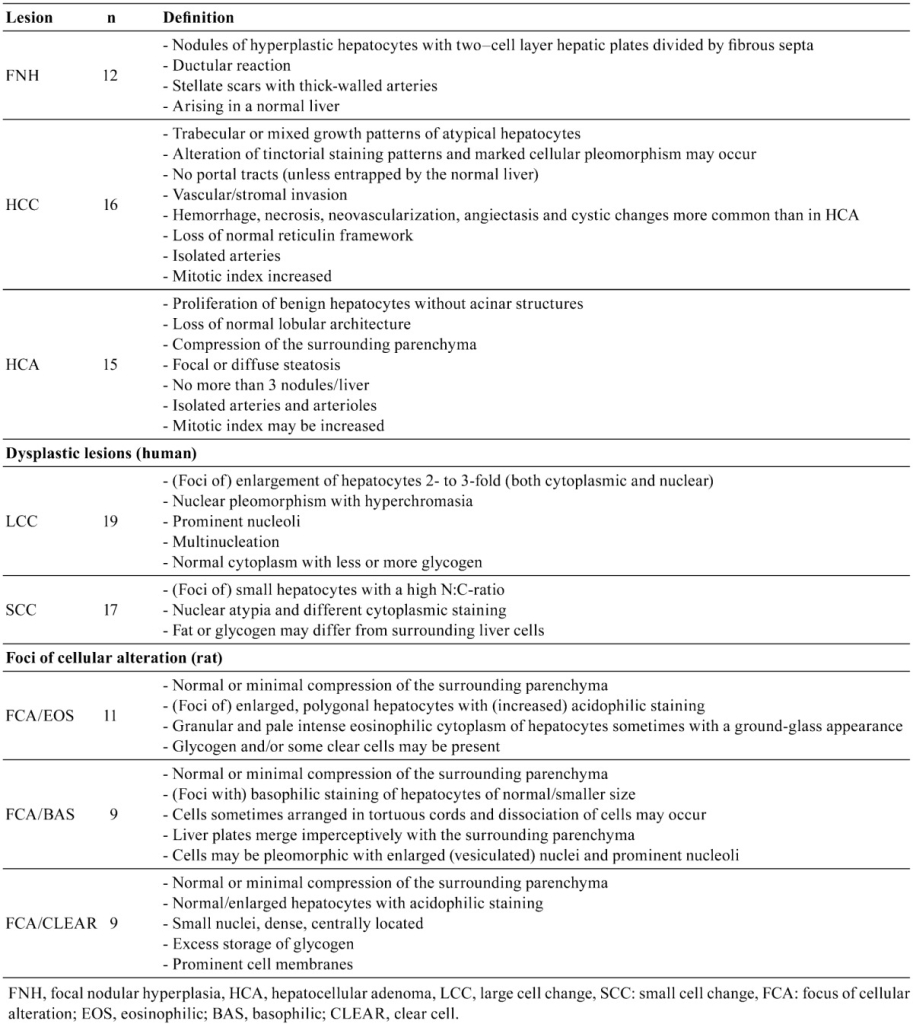
Fig. 7. Human liver. Hepatocellular adenoma with multifocal steatosis and thick-wall blood vessel (arrow). Prominent fibrosis is present on right edge of the figure. H&E.
Fig. 8. Rat liver. Hepatocellular adenoma. The adenoma is sharply demarcated with slight compression of the adjacent normal parenchyma. H&E.
Fig. 9. Human liver. A classic example of large cell change. H&E.
Fig. 10. Rat liver. Eosinophilic focus of cellular alteration consisting of enlarged hepatocytes with increased acidophilic staining compared to the surrounding hepatic parenchyma. H&E.
Fig. 11. Human liver. Small cell change comprised of a focus of small cells with an irregular margin within a cirrhotic liver. H&E.
Fig. 12. Rat liver. A basophilic focus of cellular alteration with irregular but discrete margins surrounded by more eosinophilic normal parenchymal hepatocytes. H&E.
Fig. 13. Rat liver. Clear cell focus of cellular alteration with an irregular border and comprised of hepatocytes with clear cytoplasm and a centrally located nucleus. H&E.
Discussion and Conclusion
The liver is a major target organ in preclinical toxicity and oncogenicity safety assessment studies with rodents. The significance of hepatic neoplastic findings in animal models has been questioned with regard to their predictive value, as humans appear resistant to many agents that readily produce liver tumors in rodents46. As toxicity to the liver is reported to be the second most frequent cause of drug failure due to adverse effects in clinical trials of potential drugs15,47,48, the early detection and interpretation of proliferative lesions as well as nonproliferative hepatic lesions is of vital importance. One of the safety issues after long-term administration of a xenobiotic is carcinogenicity assessment, and both early and late proliferative liver lesions might be indicative of potential hepatocarcinogenesis or carcinogenesis at other sites in humans1,16,37,49,50,51,52,53,54. The human and rat cases selected for this review were all morphologically consistent with descriptive features in texts and the peer reviewed literature using the most common and contemporary diagnostic criteria for both human and rat hepatic lesions8,22,23,24,26.
We have reviewed FCAs (eosinophilic, basophilic and clear cell foci) and compared them with the human counterparts of LCC and SCC. LCC and SCC in humans and eosinophilic and basophilic FCA in the rat showed common histomorphological characteristics (Figs. 9,10,11,12), which might be indicative of a mutual presumptive role in the process of hepatocarcinogenesis. FNH is the second most common benign lesion of the liver in humans and occurs more often in young females but can occur in both sexes of adults55,56,57,58,59,60. Cases in children have been reported61,62,63. FNH is a benign lesion, and in contrast to HCA, the risk of complications such as hemorrhage and malignant transformation is virtually absent. The more common occurrence of FNH among young women is in line with our results that show that nearly all FNHs selected for this study were obtained from women (91.7%). FNH is a rare lesion in animals64,65. Immunohistochemistry showed characteristic mild and focal cytokeratin 7 staining of hepatocytes, whereas cytokeratin 7 and cytokeratin 19 show a strong staining of bile ductules in the fibrous septa39. The majority of patients with FNH have normal liver test results and alpha-fetoprotein is mostly in the normal range66. Diagnosis of FNH may be difficult in humans if one or more major features are not prominent (central scar, ductular reaction) or if the FNH is steatotic, or has small nodules57. A clear overview of the clinical and morphological features of FNH is presented by Ferrell and Kakar24 for distinction between FNH, hepatocellular adenoma and HCC. Immunohistochemistry has also been extensively investigated and can be supportive for the differential diagnosis10,28,39,67. The current opinion regarding the etiology of this lesion is that it represents a hyperplastic and altered growth of hepatocytes surrounding a pre-existing arterial malformation in response to changes in blood flow in the parenchyma8,23,68. Treatment of symptomatic FNH in humans consists first of embolization and then resection69.
Following hepatic angioma and FNH, HCA is the third most common benign proliferative lesion in humans and is known to occur in 85% of young female patients taking oral contraceptives70,71. There is considerable overlap in morphologic features of well-differentiated hepatocellular lesions, necessitating the use of immunohistochemistry and other techniques for diagnosis39,72,73,74,75. More current diagnostic criteria distinguish four different subtypes of human HCAs based on their histological and molecular characteristics29. The human HCAs examined were all from female patients, in line with the epidemiology and common occurrence of these tumors. HCA histological features observed in the rat were similar to those in humans. The tumors showed focal fatty change/steatosis and increased numbers of mitoses and there was also normal pre-existing liver present at the periphery. In one case, multiple adenomas were observed in a rat that resembled adenomatosis as seen in humans30,70,76,77. A number of human examples showed variable amounts of steatosis as was also observed in the rat HCAs as well, occasionally accompanied by clear cell foci present in the surrounding liver in the rat. In one rat, an HCC with an infiltrative growth pattern arose within an HCA. It is known that HCA may transform into HCC in humans. Some argue that hepatocyte dysplasia probably is an essential intermediate step between HCA and HCC78. HCA is monoclonal in contrast to FNH (polyclonal) and consequently has an inherent risk for progression to HCC78. Moreover, ß-catenin-mutated HCA has an increased risk of undergoing malignant change79. Both the human and rat hepatocellular carcinomas reviewed in this study had malignant growths pattern that are typical for these tumors. Microscopically, the WHO distinguishes trabecular, acinar (pseudoglandular), scirrhous and solid forms8. Special histological subtypes, not included in this review, are the clear cell, fibrolamellar and mixed hepatocholangiocellular variants. Trabecular growth patterns were the most common in both human and rat HCCs (Figs. 3 andand 4) 4) evaluated; sometimes mixtures of trabecular, solid and acinar/pseudoglandular patterns (Figs. 5 andand 6) 6) were also present in our collection. Both human and rat HCCs showed common histopathological characteristics that are typical for these malignant liver lesions. Liver cell dysplasia (LCD) is described in liver transplants containing underlying liver disease. These dysplastic hepatocytes can frequently be observed in the cirrhotic liver19,80,81 and have been proposed to contain precancerous properties31,38,82. The cytological criteria for the diagnosis of LCD include cytoplasmic and nuclear changes, nuclear crowding or pleomorphism together with prominent nucleoli, hyperchromasia and sometimes multinucleation. The cytological features of liver cell dysplasia can strikingly mimic HCC83 suggesting it is a putative preneoplastic lesion that can precede HCC in various species1,10,41,45,84,85,86,87. The precancerous nature of both LCC and SCC with regard to progression to HCC is somewhat controversial, but some claim that either one or both of them have been associated with development of HCC31,35,38,49,82,87,88,89,90.
In rats, FCAs have likewise been designated to play a precursor role in the process of hepatocarcinogenesis as they represent a localized proliferation of hepatocytes that are phenotypically different from the surrounding liver. These FCAs occur spontaneously in aged rats and other rodents and can be induced by chemical treatment. The incidence of spontaneous foci is highest in rats and can reach nearly 100% in F344 rats by the age of 2 years91. After administration of hepatocarcinogens, their incidence, size and/or multiplicity are usually increased, and latency usually decreased25,41,86. These foci have been described in a number of animal models and are considered as precursor lesions of HCC45, but controversy still remains. Some of these foci may have autonomous growth potential and may show enzyme profiles different from the normal hepatocytes (e.g., positive for γ-glutamyl transpeptidase, α-fetoprotein, glutathione S-transferase placental form, and negative for glucose-6-phosphatase and glycogen phosphorylase). However, it has been shown that certain conditions are required for promotion and progression of initiated cells. In addition, different mechanisms of promotion by different chemicals have been demonstrated in the multistep process of carcinogenesis, and it is stated that not all foci become neoplasms91,92,93,94. Some foci of cellular alteration can even regress, and different types of foci have different potentials for developing into neoplasms17,95.
Since controversy with regard to the significance of presumptive preneoplastic liver lesions still exists, comparative research of these proliferative lesions in both rats and humans is needed.
Based on comparison of the histomorphological features of common nonneoplastic and neoplastic hepatocyte lesions of rats and humans, it is apparent that there are major similarities in diagnostic features, growth patterns, and behavior of these lesions in both species. Further study of presumptive preneoplastic lesions should help to further define their role in progression to malignancy and to provide a basis for using liver responses in rodents exposed to xenobiotics in safety assessment studies to predict potential risks to humans. Morphological similarities as illustrated in this review will be a first step to understanding their significance and relevance in human and animal liver tumor formation.
Acknowledgments
The authors thank the following people for their technical histology and editorial support: Ronald Herbert (NIEHS/NTP Archives), Keith Connely (EPL, Inc.), Emily Singletary (EPL, Inc.), Mary Ellen Sutphin (EPL, Inc.) and Suzy Tirtodikromo (Global Pathology Support). Special thanks to Nikolas Stathonikos (UMCU) for photography support.
The majority of the photomicrographs used in this document were provided courtesy of the Division of Laboratories and Pharmacy, Department of Pathology, University Medical Center Utrecht, and the National Toxicology Program Archives, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA.
This research was supported
References
1. Aleem E, Nehrbass D, Klimek F, Mayer D, Bannasch P. Upregulation of the insulin receptor and type I insulin-like growth factor receptor are early events in hepatocarcinogenesis. Toxicol Pathol. 39: 524–543 2011.
2. Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, Nevens F, Roskams T, Thorgeirsson SS. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 12: 410–416 2006.
3. Frau M, Biasi F, Feo F, Pascale RM. Prognostic markers and putative therapeutic targets for hepatocellular carcinoma. Mol Aspects Med. 31: 179–193 2010.
4. Lu X, Guo H, Molter J, Miao H, Gerber L, Hu Y, Barnes EL, Vogel H, Lee Z, Luo G, Wang B. Alpha-fetoprotein-thymidine kinase-luciferase knockin mice: A novel model for dual modality longitudinal imaging of tumorigenesis in liver. J Hepatol. 55: 96–102 2011.
5. Stefaniuk P, Cianciara J, Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 16: 418–424 2010.
6. Lopez JB. Recent developments in the first detection of hepatocellular carcinoma. Clin Biochem Rev. 26: 65–79 2005.
7. Badvie S. Hepatocellular carcinoma. Postgrad Med J. 76: 4–11 2000.
8. Bosman FT, Carneiro F, Hruban RH, Theise N.D. Tumours of the liver and intrahepatic bile ducts. In: WHO Classification of Tumours of the Digestive System, 4. International Agency for Research on Cancer, Lyon. 195-254. 2010
9. Zender L, Villanueva A, Tovar V, Sia D, Chiang DY, Llovet JM. Cancer gene discovery in hepatocellular carcinoma. J Hepatol. 52: 921–929 2010.
10. Borbath I, Leclercq IA, Sempoux C. barca-Quinones J, Desaeger C, and Horsmans Y. Efficacy of lanreotide in preventing the occurrence of chemically induced hepatocellular carcinoma in rats. Chem-Biol Interactions. 183: 238–248 2010.
11. Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md). 51: 1972–1978 2010.
12. Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. The oncologist. 15: 14–22 2010.
13. Liu Y, Wu F. Global Burden of Aflatoxin-Induced Hepatocellular Carcinoma: A Risk Assessment. Environ Health Perspect. 118: 818–824 2010.
14. Spangenberg HC, Thimme R, Blum HE. Serum markers of hepatocellular carcinoma. Semin Liver Dis. 26: 385–390 2006.
15. Tsuda H, Futakuchi M, Fukamachi K, Shirai T, Imaida K, Fukushima S, Tatematsu M, Furukawa F, Tamano S, Ito N. A medium-term, rapid rat bioassay model for the detection of carcinogenic potential of chemicals.Toxicol Pathol. 38: 182–187 2010.
16. Bannasch P, Haertel T, Qin S. Significance of Hepatic Preneoplasia in Risk Identification and Early Detection of Neoplasia. Toxicol Pathol. 31: 134–139 2003.
17. Williams GM. The significance of chemically-induced hepatocellular altered foci in rat liver and application to carcinogen detection. Toxicol Pathol. 17: 663–672 1989.
18. Terada T, Nakanuma Y. Multiple occurrence of borderline hepatocellular nodules in human cirrhotic livers: possible multicentric origin of hepatocellular carcinoma. Virchows Arch. 427: 379–383 1995.
19. Cohen C, Berson SD. Liver cell dysplasia in normal, cirrhotic, and hepatocellular carcinoma patients. Cancer. 57: 1535–1538 1986.
20. Cogliati B, Aloia TP, Bosch RV, Alves VA, Hernandez-Blazquez FJ, Dagli ML. Identification of hepatic stem/progenitor cells in canine hepatocellular and cholangiocellular carcinoma. Vet Comp Oncol. 8: 112–121 2010.
21. Park YN. Update on precursor and early lesions of hepatocellular carcinomas. Arch Pathol Lab Med. 135: 704–715 2011.
22. Goodman ZD, Ishak KG, Ferrell LD, Geisinger KR. Heptobiliary system and pancreas. In: Surgical Pathology and Cytopathology, 4th. SG Silverberg, RA DeLellis, WJ Frable, VA Livolsi, and MR Wick (eds). Elsevier, Philadelphia. 1465–1547. 2006
23. Ishak KG, Goodman ZD, Stocker JT. Tumors of the Liver and Intrahepatic Bile Ducts. Third series, Fascicle 31. Armed Forces Institute of Pathology, Washington, D.C. 2001
24. Ferrell L, Kakar S. Tumors of the liver, gallbladder, and biliary tree. In: Diagnostic Histopathology of Tumors, 3rd. CDM Fletcher (ed). Churchill Livingstone, Elsevier, Edinburgh. 417–461. 2007
25. Bannasch P, Zerban H. Tumours of the liver. In: Pathology of tumours in laboratory animals. Tumours of the rat, vol 1, 2nd. Turusov V and Mohr, U (eds) IARC scientific publications. 199–240 1990.
26. Thoolen B, Maronpot R, Harada T, Nyska A, Rousseaux C, Nolte T, Malarkey D, Kaufmann W, Küttler K, Deschl U, Nakae D, Gregson R, Vinlove M, Brix A, Singh B, Belpoggi F, Ward J. Proliferative and Nonproliferative Lesions of the Rat and Mouse Hepatobiliary System. Toxicol Pathol. 38: 5S–81S 2010.
27. Roskams T, De VR, Desmet V. ‘Undifferentiated progenitor cells’ in focal nodular hyperplasia of the liver. Histopathology. 28: 291–299 1996.
28. van Aalten SM, Verheij J, Terkivatan T, Dwarkasing RS, de Man RA, IJzermans JNM. Validation of a liver adenoma classification system in a tertiary referral centre: Implications for clinical practice. J Hepatol. 55: 120–125 2011.
29. Bioulac-Sage P, Rebouissou S, Thomas C, Blanc JF, Saric J, Sa CA, Rullier A, Cubel G, Couchy G, Imbeaud S, Balabaud C, Zucman-Rossi J. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology (Baltimore, Md). 46: 740–748 2007.
30. Rebouissou S, Imbeaud S, Jeannot E, Saric J, Balabaud C, Bioulac-Sage P, Zucman-Rossi J. Role of HNF1 inactivation in hepatocellular adenoma lipogenesis. J Hepatol. 44: S44 2006.
31. Anthony PP, Vogel CL, Barker LF. Liver cell dysplasia: a premalignant condition. J Clin Pathol. 26: 217–223 1973.
32. An CST, Petrovic LM, Reyter I, Tolmachoff T, Ferrell LD, Thung SN, Geller SA, Marchevsky AM. The application of image analysis and neural network technology to the study of large-cell liver-cell dysplasia and hepatocellular carcinoma. Hepatology (Baltimore, Md). 26: 1224–1231 1997.
33. Ganne-Carrié N, Chastang C, Chapel F, Munz C, Pateron D, Sibony M, Deny P, Trinchet JC, Callard P, Guettier C, Beaugrand M. Predictive score for the development of hepatocellular carcinoma and additional value of liver large cell dysplasia in Western patients with cirrhosis. Hepatology (Baltimore, Md). 23: 1112–1118 1996.
34. Ikeda H, Sasaki M, Sato Y, Harada K, Zen Y, Mitsui T, Nakanuma Y. Large cell change of hepatocytes in chronic viral hepatitis represents a senescent-related lesion. Hum Pathol. 40: 1774–1782 2009.
35. Le Bail B, Bernard PH, Carles J, Balabaud C, Bioulac-Sage P. Prevalence of liver cell dysplasia and association with HCC in a series of 100 cirrhotic liver explants. J Hepatol. 27: 835–842 1997.
36. Lee RG, Tsamandas AC, Demetris AJ. Large cell change (liver cell dysplasia) and hepatocellular carcinoma in cirrhosis: matched case-control study, pathological analysis, and pathogenetic hypothesis. Hepatology (Baltimore, Md). 26: 1415–1422 1997.
37. Adachi E, Hashimoto H, Tsuneyoshi M. Proliferating cell nuclear antigen in hepatocellular carcinoma and small cell liver dysplasia. Cancer. 72: 2902–2909 1993.
38. Watanabe S, Okita K, Harada T, Kodama T, Numa Y, Takemoto T, Takahashi T. Morphologic studies of the liver cell dysplasia. Cancer. 51: 2197–2205 1983.
39. Ahmad I, Iyer A, Marginean CE, Yeh MM, Ferrell L, Qin L, Bifulco CB, Jain D. Diagnostic use of cytokeratins, CD34, and neuronal cell adhesion molecule staining in focal nodular hyperplasia and hepatic adenoma. Hum Pathol. 40: 726–734 2009.
40. Maronpot RR, Montgomery CA, Jr, Boorman GA, McConnell EE. National toxicology program nomenclature for hepatoproliferative lesions of rats. Toxicol Pathol. 14: 263–273 1986.
41. Bannasch P, Enzmann H, Klimek F, Weber E, Zerban H. Significance of sequential cellular changes inside and outside foci of altered hepatocytes during hepatocarcinogenesis. Toxicol Pathol. 17: 617–628 1989.
42. Bannasch P. Hepatocellular glycogenosis and hepatic neoplasms. Toxicol Pathol. 38: 1000–1002 2010.
43. Enzmann H, Kaliner G, Watta-Gebert B, Löser E. Foci of altered hepatocytes induced in embryonal turkey liver. Carcinogenesis. 13: 943–946 1992.
44. Enzmann H, Zerban H, Löser E, Bannasch P. Glycogen phosphorylase hyperactive foci of altered hepatocytes in aged rats. Virchows Arch B. 62: 3–8 1992.
45. Libbrecht L, Desmet V, Roskams T. Review Article: Preneoplastic lesions in human hepatocarcinogenesis. Liver Int. 25: 16–27 2005.
46. Anthony PP. Liver tumours. Baillière’s Clin Gastroenterol. 2: 501–522 1988.
47. Foster JR. Spontaneous and drug-induced hepatic pathology of the laboratory Beagle dog, the cynomolgus macaque and the marmoset. Toxicol Pathol. 33: 63–74 2005.
48. Lee S, Lee HJ, Kim JH, Lee HS, Jang JJ, Kang GH. Aberrant CpG island hypermethylation along multistep hepatocarcinogenesis. Am J Pathol. 163: 1371–1378 2003.
49. Cheah PL, Looi LM, Nazarina AR, Goh KL, Rosmawati M, Vijeyasingam R. Histopathological landmarks of hepatocellular carcinoma in Malaysians. Malays J Pathol. 25: 37–43 2003.
50. Hoenerhoff MJ, Pandiri AR, Lahousse SA, Hong H-H, Ton T-V, Masinde T, Sills RC, Auerbach SS, Gerrish K, Bushel PR, Shockley KR, Peddada SD. Global gene profiling of spontaneous hepatocellular carcinoma in B6C3F1 Mice: Similarities in the molecular landscape with human liver cancer. Toxicol Pathol. 39: 678–699 2011.
51. Andersen JB, Loi R, Perra A, Factor VM, Ledda-Columbano GM, Columbano A, Thorgeirsson SS. Progenitor-derived hepatocellular carcinoma model in the rat. Hepatology (Baltimore, Md). 51: 1401–1409 2010.
52. Cohen SM. An enhanced 13-week bioassay: An alternative to the 2-year bioassay to screen for human carcinogenesis. Exp Toxicol Pathol. 62: 497–502 2010.
53. Marquardt JU, Thorgeirsson SS. Stem cells in hepatocarcinogenesis: evidence from genomic data. Semin Liver Dis. 30: 26–34 2010.
54. Samson TJ. Role of cancer stem cells in hepatocarcinogenesis. Gen Med. 3: 11 2011
55. Lefkowitch JH. Advances in hepatobiliary pathology: update for 2010. Clin Liver Dis. 14: 747–762 2010.
56. Bellamy COC. Pathology of liver tumours. Surgery (Oxford). 28: 183–188 2010.
57. Bioulac-Sage P, Laumonier H, Rullier A, Cubel G, Laurent C, Zucman-Rossi J, Balabaud C. Over-expression of glutamine synthetase in focal nodular hyperplasia: a novel easy diagnostic tool in surgical pathology. Liver Int. 29: 459–465 2009.
58. Paradis V. Benign liver tumors: an update. Clin Liver Dis. 14: 719–729 2010.
59. Blanc JF, Jeannot E, Laurent C, Sa Cunha A, Rebuissou A, Lepreux S, Le Bail B, Troutte H, Saric J, Balabaud C, Zucman-Rossi J, Bioulac-Sage P. 232 Focal nodular telangiectasia (FNT) of the liver: Pathological and molecular characterization. J Hepatol. 40: 74 2004.
60. Kayhan A, Venu N, Lakadamyali H, Jensen D, Oto A. Multiple progressive focal nodular hyperplasia lesions of liver in a patient with hemosiderosis. World J Radiol. 2: 405–409 2010.
61. Farruggia P, Alaggio R, Cardella F, Tropia S, Trizzino A, Ferrara F, D’Angelo P. Focal nodular hyperplasia of the liver: an unusual association with diabetes mellitus in a child and review of literature. Ital J Pediatr. 36: 41 2010.
62. Sugito K, Uekusa S, Kawashima H, Furuya T, Ohashi K, Inoue M, Ikeda T, Koshinaga T, Tomita R, Mugishima H, Maebayashi T. The clinical course in pediatric solid tumor patients with focal nodular hyperplasia of the liver. Int J Clin Oncol. 16: 482–487 2011.
63. Towbin AJ, Luo GG, Mo JQ, Mo JQ. Focal nodular hyperplasia in children, adolescents, and young adults. Pediatr Radiol. 41: 341–349 2011.
64. Fujishima J, Satake S, Furukawa T, Kurokawa C, Kodama R, Moriyama A, Sasaki Y, Kamimura Y, Maeda H. Focal nodular hyperplasia in the livers of cynomolgus macaques (Macaca fascicularis). J Toxicol Pathol. 24: 125–129 2011.
65. Sumiyoshi M, Sakanaka M, Kimura Y. Chronic intake of a high-cholesterol diet resulted in hepatic steatosis, focal nodular hyperplasia and fibrosis in non-obese mice. Brit J Nutr. 103: 378–385 2010.
66. Mneineh W, Paradis V. High serum level of alpha-fetoprotein in focal nodular hyperplasia of the liver. Pathol Int. 61: 491–494 2011.
67. Zhu ZW, Friess H, Wang L., bou-Shady M, Zimmermann A, Lander AD, Korc M, Kleeff J, and Büchler MW. Enhanced glypican-3 expression differentiates the majority of hepatocellular carcinomas from benign hepatic disorders. Gut. 48: 558–564 2001.
68. Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology (Baltimore, Md). 5: 1194–1200 1985.
69. Arts CH. van HR, de Kort GA, and Moll FL. Inferior caval vein thrombosis owing to compression of focal nodular hyperplasia: surgical resection after shrinkage by hepatic artery embolization. Vascular. 18: 53–58 2010.
70. Maillette De Buy Wenniger L, Terpstra V, Beuers U. Focal nodular hyperplasia and hepatic adenoma: Epidemiology and pathology. Dig Surg. 27: 24–31 2010.
71. Bioulac-Sage P, Laumonier H, Laurent C, Zucman-Rossi J, Balabaud C. Hepatocellular adenoma: what is new in 2008. Hepatol Int. 2: 316–321 2008.
72. Di C. I, Pulvirenti E, Toro A, and Priolo GD. Adenoma or atypical hepatic focal nodular hyperplasia: role of preoperative imaging and laparoscopic treatment. Surg Lapar Endos & Percut Techn. 20: e105–e109 2010.
73. Bioulac-Sage P, Balabaud C, Zucman-Rossi J. Focal nodular hyperplasia, hepatocellular adenomas: past, present, future. Gastroenterol Clin Biol. 34: 355–358 2010.
74. Bioulac-Sage P, Cubel G, Balabaud C, Zucman-Rossi J. Revisiting the pathology of resected benign hepatocellular nodules using new immunohistochemical markers. Semin Liver Dis. 31: 91–103 2011.
75. Shafizadeh N, Kakar S. Diagnosis of well-differentiated hepatocellular lesions: role of immunohistochemistry and other ancillary techniques. Adv Anat Pathol. 18: 438–445 2011.
76. Bioulac-Sage P, Balabaud C, Zucman-Rossi J. Subtype classification of hepatocellular adenoma. Dig Surg. 27: 39–45 2010.
77. Reznik Y, Dao T, Coutant R, Chiche L, Jeannot E, Clauin S, Rousselot P, Fabre M, Oberti F, Fatome A, Zucman-Rossi J, Bellanne-Chantelot C. Hepatocyte nuclear factor-1 alpha gene inactivation: cosegregation between liver adenomatosis and diabetes phenotypes in two maturity-onset diabetes of the young (MODY)3 families. Clin Endocrinol Metab. 89: 1476–1480 2004.
78. Farges O, Dokmak S. Malignant transformation of liver adenoma: an analysis of the literature. Dig Surg. 27: 32–38 2010.
79. Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S, Wendum D, Chiche L, Fabre M, Mellottee L, Laurent C, Partensky C, Castaing D, Zafrani ES, Laurent-Puig P, Balabaud C, Bioulac-Sage P. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology (Baltimore, Md). 43: 515–524 2006.
80. Kobayashi M, Ikeda K, Hosaka T, Sezaki H, Someya T, Akuta N, Suzuki F, Suzuki Y, Saitoh S, Arase Y, Kumada H. Dysplastic nodules frequently develop into hepatocellular carcinoma in patients with chronic viral hepatitis and cirrhosis. Cancer. 106: 636–647 2006.
81. Lefkowitch JH, Apfelbaum TF. Liver cell dysplasia and hepatocellular carcinoma in non-A, non-B hepatitis. Arch Pathol Lab Med. 111: 170–173 1987.
82. Libbrecht L, Craninx M, Nevens F, Desmet V, Roskams T. Predictive value of liver cell dysplasia for development of hepatocellular carcinoma in patients with non-cirrhotic and cirrhotic chronic viral hepatitis. Histopathology. 39: 66–73 2001.
83. Tao LC. Oral contraceptive-associated liver cell adenoma and hepatocellular carcinoma. Cytomorphology and mechanism of malignant transformation. Cancer. 68: 341–347 1991.
84. Podda M, Roncalli M, Battezzati PM, Borzio M, Bruno S, Servida E, Coggi G. Liver-cell dysplasia and hepatocellular carcinoma. Ital J Gastroenterol. 24: 39–42 1992.
85. Enzmann H, Kuhlem C, Kaliner G, Löser E, Bannasch P. Rapid induction of preneoplastic liver foci in embryonal turkey liver by diethylnitrosamine. Toxicol Pathol. 23: 560–569 1995.
86. Maronpot RR, Pitot HC, Peraino C. Use of rat liver altered focus models for testing chemicals that have completed two-year carcinogenicity studies. Toxicol Pathol. 17: 651–662 1989.
87. Koo JS, Kim H, Park B, Ahn S, Han KH, Chon C, Park C, Park Y. Predictive value of liver cell dysplasia for development of hepatocellular carcinoma in patients with chronic hepatitis B. J Clin Gastroenterol. 42: 738–743 2008.
88. Borzio M, Bruno S, Roncalli M, Mels GC. Liver cell dysplasia is a major risk factor for hepatocellular carcinoma in cirrhosis: a prospective study. Gastroenterology. 108: 812 1995.
89. Park YN, Roncalli M. Large liver cell dysplasia: a controversial entity. J Hepatol. 45: 734–743 2006.
90. Rubin EM, DeRose PB, Cohen C. Comparative image cytometric DNA ploidy of liver cell dysplasia and hepatocellular carcinoma. Mod Pathol. 7: 677–680 1994.
91. Narama I, Imaida K, Iwata H, Nakae D, Nishikawa A, Harada T. A review of nomenclature and diagnostic criteria for proliferative lesions in the liver of rats by a working group of the Japanese Society of Toxicologic Pathology. J Toxicol Pathol. 16: 1–17 2003.
92. Popp JA, Goldsworthy TL. Defining foci of cellular alteration in short-term and medium-term rat liver tumor models. Toxicol Pathol. 17: 561–568 1989.
93. Harada T, Maronpot RR, Morris RW, Stitzel KA, Boorman GA. Morphological and stereological characterization of hepatic foci of cellular alteration in control Fischer 344 rats. Toxicol Pathol. 17: 579–593 1989.
94. Pitot HC, Campbell HA, Maronpot R, Bawa N, Rizvi TA, Xu YH, Sargent L, Dragan Y, Pyron M. Critical parameters in the quantitation of the stages of initiation, promotion, and progression in one model of hepatocarcinogenesis in the rat. Toxicol Pathol. 17: 594–611 1989.
95. Enzmann H, Bannasch P. Non-persisting early foci of altered hepatocytes induced in rats by N-nitrosomorpholine. J Cancer Res Clin Oncol. 114: 30–34 1988.