Highlights
- • Bacterial cyclodextrin glucanotransferase (CGTase) is used to produce a water soluble form of glycosylated isoquercitrin.
- • Genotoxicity battery on CGTase and sodium sulfate negative for mutations and DNA damage.
- • No evidence of systemic toxicity in 90-day rat toxicity study of CGTase
Introduction
Microbiologically derived cyclodextrin glucanotransferase (CGTase) is used commercially as a processing agent in manufacture of food, pharmaceuticals, and cosmetics. Its toxic potential was evaluated in anticipation of use in the production ofalpha-glycosyl isoquercitrin, a water-soluble form of quercetin.
Methods
Following OECD guidelines, CGTase, produced by Bacillus pseudalcaliphilus DK-1139, was evaluated in a genotoxicity battery consisting of a bacterial reverse mutation assay, an in vitro micronucleus (MN) assay and MN and comet assays using B6C3F1 male and female mice. These same genotoxicity assays were also conducted for sodium sulfate, a contaminant of CGTase preparation. In a 90-day Sprague Dawley rat toxicity study, CGTase was administered by gavage in water at daily doses of 0, 250, 500, and 1000 mg/kg/day.
Results
CGTase did not induce mutations with or without metabolic activation in the bacterial reverse mutation assay. Formation of micronuclei was not induced in either in vitro or in vivo MN assays with or without metabolic activation. No induction of DNA damage was detected in male or female mouse liver, stomach, or duodenum in the comet assay. Sodium sulfate also tested negative in these same genotoxicity assays. In the 90-day repeated dose rat study there were no treatment-related adverse clinical or pathological findings.
Conclusion
The genotoxicity assays and repeated dose toxicity study support the safe use of CGTase in production of alpha-glycosyl isoquercitrin.
Chemical compounds studied in this article
- Sodium sulfate (PubChem CID: 24436)
Abbreviations
- CGTase, cyclodextrin glucanotransferase;
- EMIQ, enzymatically modified isoquercitrin;
- MN, micronuclei;
- MN-RET, micronucleated reticulocytes;
- MN-NCE, micronucleated normochromatic erythrocytes;
- GLP, good laboratory practice;
- NOAEL, no observed adverse effect level;
- OECD, Organization for Economic Co-operation and Development;
- EFSA, European Food Safety Authority
Keywords
- Micronucleus assay;
- Comet assay;
- Enzymatically modified isoquercitrin (EMIQ);
- Food additive;
- Flavonol;
- Sodium sulfate
1. Introduction
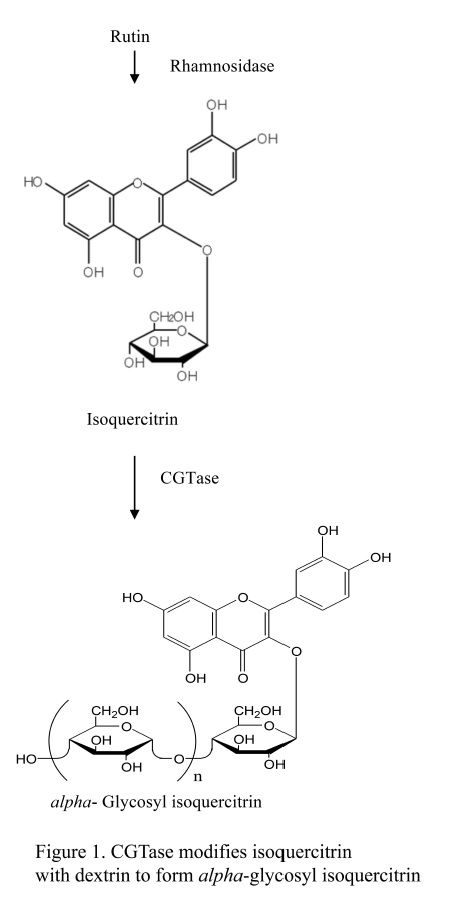
Cyclodextrin glucanotransferase (CGTase) is one of several starch-hydrolyzing extracellular enzymes produced by many bacteria to convert large molecules to utilizable small molecules for growth. CGTase, a member of the alpha-amylase superfamily, catalyzes cleavage of glycosidic bonds between carbohydrates and/or non-carbohydrate moieties. As an amylolytic enzyme, CGTase is used commercially in manufacture of food, pharmaceuticals, and cosmetics ( Hedges, 1998; Thompson, 1997). Our interest is in use of CGTase in synthesis of glycosylated molecules such as alpha-glycosyl isoquercitrin (EMIQ). CGTase catalyzes addition of one or more glucose units to isoquercitrin to produce EMIQ ( Fig. 1); during processing, this step is followed by heat inactivation of the CGTase. Even though not present in the final product, food enzymes are subject to safety evaluation according to EFSA and current Japanese regulations (EFSA, 2009; EFSA, 2014). In the present study we provide a safety evaluation of CGTase, produced by Bacillus pseudalcaliphilus DK-1139, using a battery of genotoxicity assays and a 90-day repeated dose toxicity study in Sprague Dawley rats.
2. Methods
2.1. Chemical analysis
All genotoxicity assays were conducted according to OECD guidelines and were Good Laboratory Practice (GLP)-compliant. Samples removed from the top, middle, and bottom fractions of each chemical formulation were submitted for analytical testing (Alera Laboratories, LLC, Durham, North Carolina, USA). Most analyzed formulation concentrations were greater than 15% of the nominal dose or were within the ±15% acceptance criteria. The few formulations that were lower than 15% of the nominal dose were low or mid-range doses that were not used as benchmark doses to determine overall assay responses. CGTase (Composition = 85% carbohydrate, 0.3% protein, 0.7% lipid, 12.1% water, 0.26% sodium sulfate, ≤ 2 ppm lead, ≤0.5 ppm arsenic, and 1.0% total organic solvent; CAS No. 9030-09-5; San-Ei Gen F.F.I., Inc., Osaka, Japan) was prepared in sterile deionized water and stored between 0 and −30 °C and away from light. The neat test article was determined to be stable, based on enzyme titer, for a period of three months with storage at approximately −15 °C. Continued stability is inferred from repeated formulation analyses in which the formulation concentrations, calculated from enzyme activity measurements, routinely exceeded nominal concentrations (Alera Labs, 2014). Sodium sulfate (99.7% pure; Tomita Pharmaceutical Co. Ltd., Tokushima, Japan) prepared in sterile deionized water and stored at 1–10 °C and away from light is stable for at least 8 days (Alera Labs, 2014). Sodium sulfate, present at low levels in preparations of CGTase, was also tested in the genotoxicity battery.
2.2. Bacterial reverse mutation assay
GLP mutagenicity assays of CGTase and sodium sulfate, with and without metabolic activation, were conducted as described previously (Ames et al., 1975, Maron and Ames, 1983 and Mortelmans and Zeiger, 2000) using the following five Salmonella and E. colistrains as prescribed in the guideline for the bacterial reverse mutation assay ( OECD, 1997a): TA98, TA100, TA97a, TA1535, and E. coli WP2 uvrA pKM101. All strains (Moltox, Inc., Boone, NC) were checked for maintenance of genetic markers prior to the study. Nominal concentrations of CGTase ranged from 25 to 5000 μg/plate. Concentrations of sodium sulfate ranged from 250 to 5000 μg/plate. Metabolic activation was provided using phenobarbital/benzoflavone-induced rat liver S9 (Moltox, Inc., Boone, NC) with cofactors (Regensys™ NADPH Regeneration System Reagents, Moltox, Inc., Boone, North Carolina, USA). The composition of the S9 mix was: 10% S9, 8 mM MgCl2, 32.6 mM KCl, 4.7 mM glucose-6-phosphate, 4 mM NADP, and 0.1 M phosphate buffer. Cytotoxicity was assessed by changes in the background bacterial lawn (i.e., enhanced or absent lawn), reduced revertant counts, presence of microcolonies, and/or absence of colonies. Strain specific positive controls tested without metabolic activation were 2-nitrofluorene, 3 μg (TA98), sodium azide, 1 μg (TA100 and TA1535), ICR191, 0.25 μg (TA97a), and 4-nitroquinoline-N-oxide, 0.25 μg (E. coli WP2). Benzo
2.3. In vitro micronucleus (MN) assay
The GLP in vitro MN assay was conducted in compliance with OECD TG 487 (OECD 2014a). Human TK6 cells (ATCC, Manassas, VA) were cultured and maintained in RPMI 1640 medium containing 10% heat inactivated horse serum plus 1.0% Pluronic F-68, 0.5% sodium pyruvate, and antibiotics (penicillin at 20 Units/mL and streptomycin at 20 μg/mL) at 37 °C, with 6% CO2. The normal cell cycle time of these cells is approximately 12 hours. Metabolic activation was provided using phenobarbital/benzoflavone-induced rat liver S9 (Moltox, Boone, NC) with added Regensys™ cofactors (Moltox, Boone, NC) at a final concentration of 1% S9. The composition of the S9 mix was: 10% S9, 8 mM MgCl2, 32.6 mM KCl, 4.7 mM glucose-6-phosphate, 4 mM NADP, and 0.1 M phosphate buffer. Triplicate cultures of exponentially growing cells seeded at 0.40 × 106 cells/mL in 12-well plates were exposed to CGTase, sodium sulfate, or controls for approximately four hours in the presence of metabolic activation (+S9) and approximately 24–29 hours in the absence of metabolic activation (-S9). Cyclophosphamide (Sigma-Aldrich, St. Louis, MO) and vinblastine (Sigma-Aldrich, St. Louis, MO) were used as the positive controls with and without metabolic activation, respectively. On the basis of preliminary tests, the concentrations of CGTase selected for testing were 6000, 5000, 4000, 3000, 2000, and 1000 μg/mL of CGTase for approximately 4 hours with S9 and 24 hours without S9. Concentrations of sodium sulfate selected were 5000, 1667, 556, 185, 61.7, and 20.6 μg/mL for 4 hours with S9 and 24 hours without S9. At the end of the culture period, cells were analyzed for cytotoxicity and micronucleus induction by flow cytometry using the In Vitro MicroFlow™ kit (Litron Laboratories, Rochester, NY) according to manufacturer’s instructions. Unless limited by cytotoxicity, 20,000 cells from each sample were analyzed for the frequency of micronuclei (MN) using a FACSCalibur™ dual-laser bench top system (Becton Dickinson Biosciences, San Jose, CA).
Cytotoxicity was measured as relative increase in cell count or as relative survival of cells in vehicle control cultures compared to cells in treated cultures using ratios of counted nuclei to counted beads (inert latex microspheres added to each sample). Higher nuclei to bead ratios correspond to greater cell survival. Greater emphasis was placed on relative survival since this flow cytometry-based measurement may provide a more sensitive assessment of cytotoxicity, enhancing assay specificity (Avlasevich et al., 2011). As recommended by OECD test guideline #487 (OECD, 2014a), concentrations inducing greater than 60% cytotoxicity were excluded from the analysis. An increase in MN frequency ≥3-fold over the mean MN frequency in the concurrent vehicle control was considered a positive response.
2.4. Animal husbandry
For the combined in vivo MN and comet assays male and female B6C3F1 mice (Charles River Laboratories International, Inc., Raleigh, NC) were 8–10 weeks of age at the time of treatment. Animals were housed in polycarbonate cages with absorbent hardwood bedding in an AAALAC-accredited specific pathogen free facility with a 12 hour light/12 hour dark cycle. Certified Purina Pico Chow No. 5002 (Ralston Purina Co., St. Louis, MO) and water were provided ad libitum. For the 90-day toxicity study male and female Hsd:Sprague Dawley rats (Harlan Laboratories, Inc., Frederick, MD) were housed one per cage in polycarbonate cages with heat-treated hardwood bedding (Northeastern Products Corp., Warrensburg, NY) and fed Purina Certified 5002 Meal Diet (Ralston Purina Co., St. Louis, MO) ad libitum. These studies were approved by the ILS, Inc. Institutional Animal Care and Use Committee, and all procedures were completed in compliance with the Animal Welfare Act Regulations, 9CFR 1–4, and theGuide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 2011).
2.5. In vivo MN/Comet assay experimental design
GLP studies were conducted in compliance with OECD TG 474 (OECD, 2014b) and in general accordance with OECD TG 489 (OECD, 2014c). Dose range finding studies were conducted to identify appropriate dose levels. Male and female mice were administered CGTase or sodium sulfate via oral gavage up to and including dose levels of 2000 mg/kg/day (test guideline limit dose) for three consecutive days. No marked changes in body weights were measured nor adverse clinical observations found in mice administered either chemical and there were no abnormal gross necropsy organ observations. Therefore, for the definitive studies, male and female B6C3F1 mice (5 animals/dose group) were administered CGTase or sodium sulfate at 1000, 1500, or 2000 mg/kg/day, vehicle (deionized water), or the positive control compound, ethyl methanesulfonate (EMS) (Sigma-Aldrich, St. Louis, MO) in 0.9% saline (Ricca Chemical Company, Arlington, TX) at 150 mg/kg/day, daily for three days by oral gavage. Three hours after the final dose, peripheral blood was collected for flow cytometric analysis of MN, and liver, duodenum, and stomach tissues were collected, frozen in liquid nitrogen, and stored at ‐80 °C until analysis by the comet assay (Recio et al., 2012).
2.6. Erythrocyte micronucleus assay
Peripheral blood samples were processed for flow cytometric evaluation of micronucleated reticulocytes (MN-RET) as described previously (Witt et al., 2008). Briefly, cells were fixed and labeled using a MicroFlowPLUS Kit (Litron Laboratories, Rochester, NY) according to manufacturer’s directions and analyzed using a FACSCalibur flow cytometer (Becton Dickinson, Sunnyvale, CA). For each peripheral blood sample, 20,000 RET were analyzed to determine the frequency of MN-RET. More than 106 mature normochromatic erythrocytes were enumerated concurrently during MN-RET analysis, and the percentage of RET (%RET) among total erythrocytes was calculated as a measure of bone marrow toxicity.
2.7. Comet assay
For each animal, a portion of the left lobe of the liver was placed into a tube containing cold mincing solution [Mg++ and Ca++ free Hank’s Balanced Salt Solution (Invitrogen, Carlsbad, CA) containing 10% v/v dimethylsulfoxide (DMSO), and 20 mM disodium ethylenediaminetetraacetic acid (Na2EDTA) pH 7.4–7.7] and rapidly minced. A portion of the duodenum proximal to the stomach was removed, flushed with mincing solution, and kept cold and moist with mincing solution. The tissue was trimmed and a small section placed in a microcentrifuge tube containing mincing solution and rapidly minced. The stomach was cut open and washed free from food using cold mincing buffer. The glandular stomach was placed into cold mincing buffer and incubated on ice for 15–30 minutes, then the surface epithelium was gently scraped two times using a scalpel blade. This layer was discarded and the gastric mucosa rinsed with cold mincing buffer. The stomach epithelium was carefully scraped 4–5 times in mincing solution with a scalpel blade to release the cells. The mincing solution containing released epithelial cells was transferred to microfuge tubes. Tissue samples were flash frozen in liquid nitrogen and stored at −80 °C until processed (Recio et al., 2012). Additional portions of each tissue were fixed in 10% neutral buffered formalin (NBF) for at least 24 hours, trimmed, and paraffin embedded for possible histopathology assessment of cytotoxicity in the case of a positive response in the comet assay.
For processing, cells were partially thawed in a warm water bath and placed on ice until slide preparation. Cell samples were empirically diluted with 0.5% low melting point agarose (Lonza, Walkersville, MD) dissolved in Dulbecco’s phosphate buffer (Ca++, Mg++ and phenol free) at 37 °C, layered onto each well of a 2-well CometSlide™ (Trevigen, Gaithersburg, MD), and immersed in cold lysing solution (2.5 M NaCl, 100 mM Na2EDTA, 10 mM Tris, pH 10, containing freshly added 10% DMSO and 1% Triton X–100) overnight. After rinsing in 0.4 M Trizma base (pH 7.5), slides were treated with alkali (300 mM NaOH, 1 mM Na2EDTA, pH > 13) for 20 minutes, then electrophoresed at 4 °C for 20 minutes at ∼1.0 V/cm, 300 mA. After electrophoresis, slides were neutralized with 0.4 M Trizma base (pH 7.5) for 5 minutes, incubated for 5 minutes in ice-cold 100% ethanol (Pharmco-AAPER, Shelbyville, KY) and allowed to air-dry. Slides were stored at room temperature in a desiccator until stained and scored. After staining slides with SYBR Gold™ (Molecular Probes, Invitrogen, Carlsbad, CA), 100 cells were scored per sample at 200x magnification without knowledge of sample identity using Comet Assay IV Imaging Software¸ Version 4.3.1 (Perceptive Instruments, Ltd., Suffolk, UK). The extent of DNA migration was characterized using the % tail DNA endpoint measurement (intensity of all tail pixels divided by the total intensity of all pixels in the comet, expressed as a percentage). NaCl, Na2EDTA, Triton X–100, and Trizma base were purchased from Sigma-Aldrich (St. Louis, MO); NaOH and DMSO were purchased from Fisher Scientific (Pittsburgh, PA).
2.8. 90-day repeat dose toxicity study
This GLP-compliant 90-day rat study was conducted following OECD TG 408 (OECD, 1998). Ten males and ten females were allocated to each of four designated dose groups. The animals were administered one of three dose levels (250, 500, or 1000 mg/kg/day) of CGTase or deionized water for 90 days via oral gavage. Cage-side observations were performed daily and body weight measurements and clinical observations performed weekly. Ophthalmological examinations were performed prior to exposure to CGTase and again within a week of termination. Following 11 weeks of exposure, neurotoxicity screening was performed including measurement of motor activity and a functional observation battery as defined in OECD TG 424 (OECD, 1997b).
After 90 days of CGTase administration, animals were humanely euthanized. Prior to termination, urine was collected and submitted for analysis. A full screen necropsy was performed and tissue weights were collected for adrenals, brain, epididymides, heart, kidneys, liver, lungs, ovaries, pituitary, prostate, salivary glands, seminal vesicles, spleen, testes, thymus, thyroids with parathyroids and uterus with cervix. The following tissues were fixed in 10% NBF (with the exception of eyes and testes that were fixed in modified Davidson’s fixative), embedded in paraffin, sectioned, and microscopically evaluated: adrenals, aorta, bone (sternum and femur), bone/bone marrow (sternum and femur), brain (cerebrum, cerebellum, and medulla/pons), cecum and colon, corpus and cervix uteri, epididymides, esophagus, eyes, heart, kidneys, liver, intestines (small and large including Peyer’s patches), lungs (with main-stem bronchi), lymph nodes (mesenteric and mandibular), mammary glands, muscle (skeletal), nasal cavity, ovaries, oviducts, pancreas, pituitary, prostate, salivary glands, sciatic nerve, seminal vesicles, skin, spinal cord (3 locations: cervical, mid-thoracic, lumbar), spleen, stomach, testes, thymus (thymic region), thyroids/parathyroid(s), tongue, trachea, uterus, urinary bladder, vagina, Zymbal’s glands. Blood, collected by vena cava puncture immediately following termination, was submitted for hematology, clinical chemistry, and hormone determinations (See Tables 5–7 for lists of measurements).
2.9. Statistical analyses
For the in vivo MN/comet assay, body weight, MN-RET and RET frequency data, and DNA damage data were analyzed using Statistical Analysis System version 9.2 (SAS Institute, Cary, NC). Homogeneity of the data was assessed using the Levene’s test with a 95% confidence level. Data were then analyzed using a one-way analysis of variance (ANOVA) and treated groups compared to the appropriate control group using Dunnett’s test. Dose-dependent changes were evaluated using linear regression. Data that were not homogeneous and normally distributed were analyzed using the appropriate non-parametric one-tailed Dunn’s test. Dose-dependent changes were evaluated using an appropriate non-parametric trend test (Jonckheere’s test). For all in vivo endpoints, a one-tailed t-test was used to verify a positive response to the reference compound EMS (p < 0.05). Criteria for a positive result in the MN and comet assays were at least one statistically significant dose group (p < 0.05), a dose group falling outside the range of laboratory historical control data, and a statistically significant trend test (p < 0.05). A test was considered equivocal if only one or two of these conditions were met (OECD, 2014a, b).
Endpoints in the functional observation battery using interval scales were evaluated for homogeneity using Levene’s test. A non-significant result (p > 0.001) indicated that an assumption of homogeneity of variance was appropriate, and the data were compared using an ANOVA test with groups administered CGTase compared to the vehicle control group using Dunnett’s test. If Levene’s test was significant (p ≤ 0.001), the ANOVA test was not appropriate and the Kruskal-Wallis test was used to analyze the data; in the event of a significant result (p ≤ 0.05), Dunn’s test was used to compare the groups administered CGTase with the control group. Endpoints in the functional observation battery using graded or count scales were analyzed using a non-parametric strategy. When 75% or fewer of the scores in all the groups were tied, the Kruskal-Wallis test was used to analyze the data; in the event of a significant result (p ≤ 0.05), Dunn’s test was used to compare the CGTase groups with the control group. When more than 75% of the scores in any group were tied, Fisher’s Exact test was used to compare the proportion of ties in the groups. Endpoints in the functional observation battery using descriptive or quantal scales were analyzed using the Fisher’s Exact test.
Data from the motor activity test, with repeated measurements within a session, were analyzed using an ANOVA with Repeated Measures. A significant effect (p ≤ 0.05) in that test can appear as effect of dose (a difference between groups in the total across all measurements in a session) or as an interaction between dose and time (a difference between groups at specific measurement periods).
3. Results
3.1. Results of the bacterial reverse mutation assays
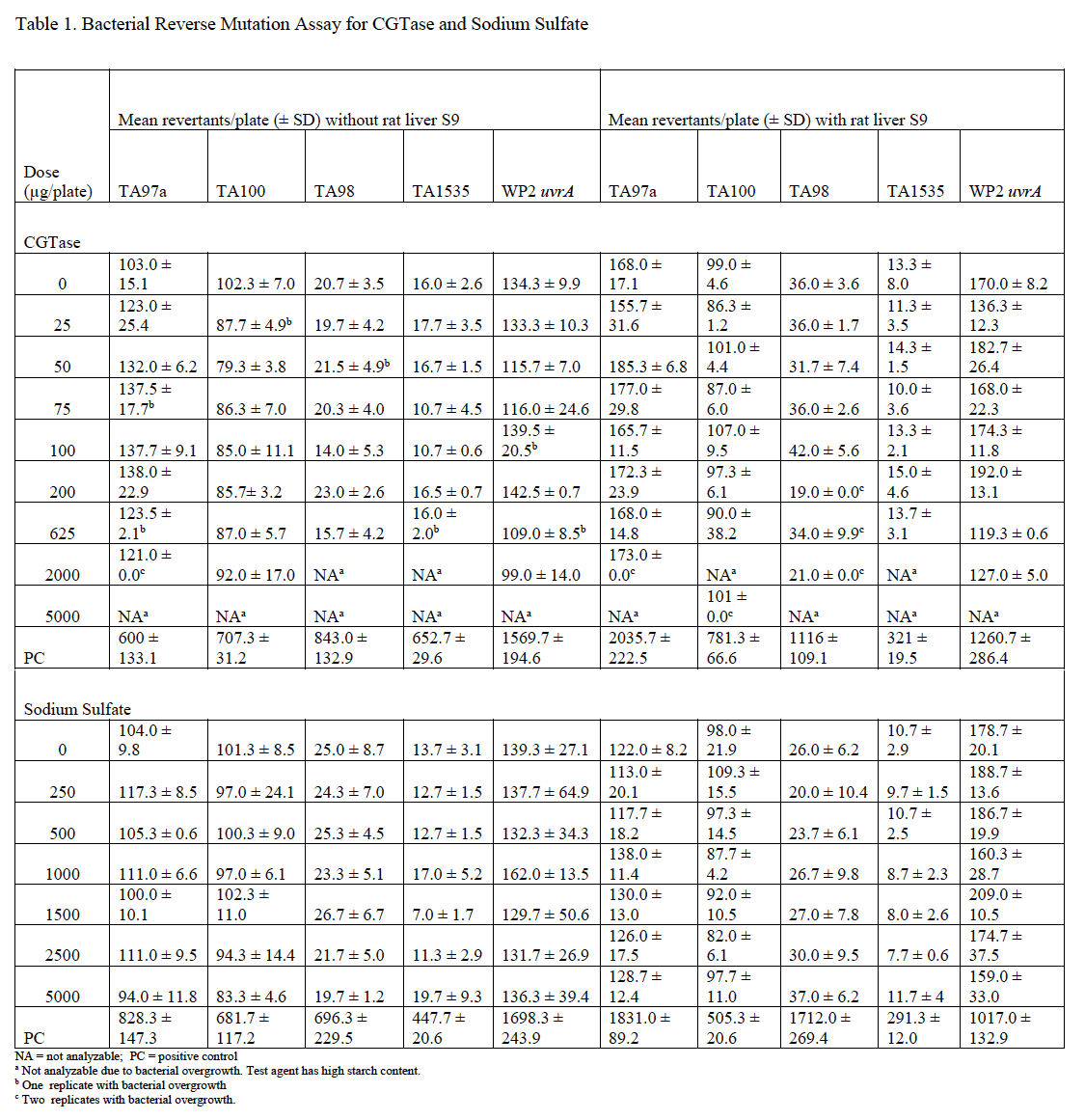
Mutagenicity assays were conducted to assess the potential of CGTase and sodium sulfate to induce gene mutations in bacteria using the pre-incubation method. CGTase caused bacterial overgrowth at several doses in all strains tested. Precipitate was observed in all assay tubes and decreased with decreasing dose of test article, but the precipitate did not interfere with automatic plate counting. CGTase was not cytotoxic and was not mutagenic at analyzable doses in any strain used, either without or with metabolic activation. Therefore, CGTase is considered to be negative for mutagenicity in the bacterial reverse mutagenicity assay under the conditions tested.
Sodium sulfate was not cytotoxic, did not precipitate, and was not mutagenic in any strain used, either without or with metabolic activation. Therefore, sodium sulfate is considered negative for mutagenicity in the bacterial reverse mutagenicity assay under the conditions tested. Average plate counts for each set of replicate plates for both CGTase and sodium sulfate are provided in Table 1.
3.2. Results of the in vitro MN assays
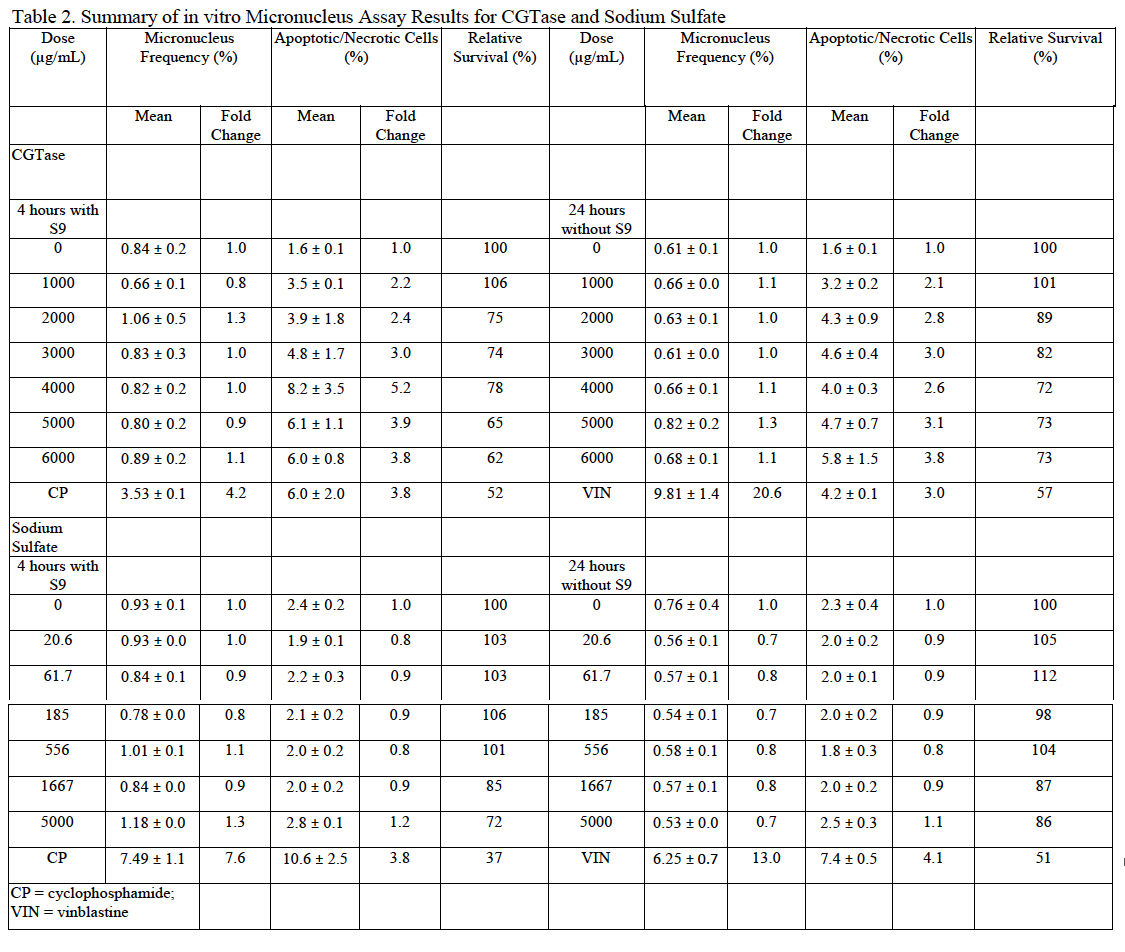
MN are identified by flow cytometry using a combination of characteristics of size (as measured by light scatter) and fluorescence (based on differential staining) that differentiates debris and necrotic and apoptotic cells from healthy cells containing micronuclei (Avlasevich et al., 2006 and Bryce et al., 2007). CGTase was slightly cytotoxic to cultured TK6 cells at concentrations of approximately 4000 μg/mL and greater ±S9; however, cytotoxicity at all tested concentrations was less than 50%. Precipitation was observed at all tested doses. No pH changes were noted based on media color change. Because the induction of micronuclei was less than 3-fold higher than the vehicle control at all doses both with and without S9, the overall response was considered negative. These data indicate that, under the conditions tested in this study, CGTase is not excessively cytotoxic to cultured TK6 cells and does not induce micronuclei in this test system. MN frequency and cell viability data for cultures exposed to CGTase are summarized in Table 2.
Sodium sulfate was not toxic to cultured TK6 cells at the doses tested. Precipitation was not observed at any tested dose. No pH changes were noted based on media color change. Because the induction of micronuclei was less than 3-fold higher than the vehicle control at all doses both with and without S9, the overall response was considered negative. These data indicate that, under the test conditions used in this study, sodium sulfate is non-toxic to cultured TK6 cells and does not induce micronuclei in this test system. MN frequency and cell viability data for cultures exposed to sodium sulfate are summarized in Table 2.
3.3. Results of the in vivo MN/Comet assays
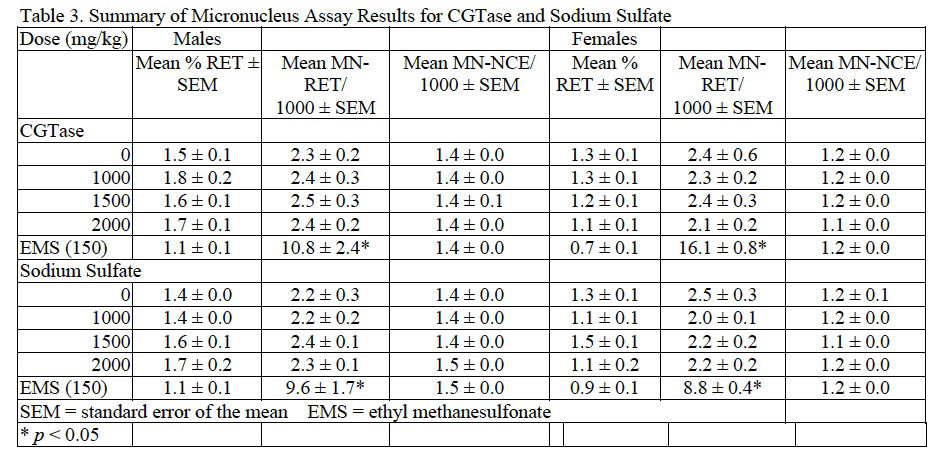
Based on the results of preliminary dose setting studies, combined MN/comet assays were conducted in which male and female B6C3F1 mice were administered CGTase or sodium sulfate at 1000, 1500, and 2000 mg/kg/day for 3 consecutive days. No abnormal clinical signs related to chemical administration were noted during the course of the studies for CGTase, sodium sulfate, or the EMS positive control. Following CGTase administration, a decreasing trend in body weight gain without an effect in final body weight was seen in male mice but not regarded as biologically relevant. There was no suggestion of bone marrow toxicity in mice administered CGTase up to 2000 mg/kg/day as reflected by the lack of an effect on RET frequency. The MN-RET frequency in mice administered CGTase was not significantly different from concurrent vehicle controls; therefore, the MN assay was clearly negative in both male and female mice. Results for the MN assay of CGTase are summarized in Table 3.
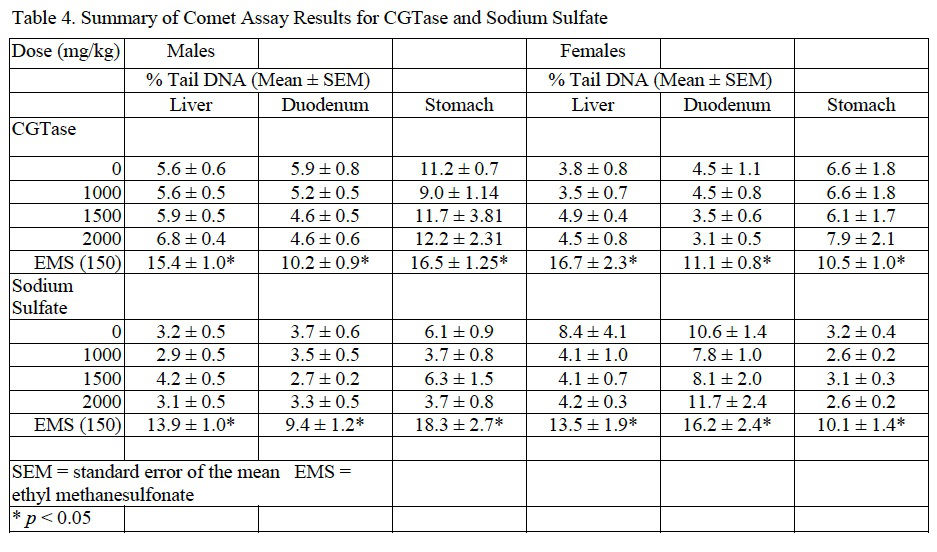
Induction of DNA damage was not observed in liver, duodenum, or stomach of male or female mice exposed to CGTase. Moreover, there was no associated dose-related trend and the data for the CGTase (and vehicle control) dose groups were within the 95% confidence interval of laboratory historical vehicle control data. Therefore, the comet assay conducted in mice is considered negative. Comet assay results for CGTase are presented in Table 4.
In mice administered sodium sulfate there were no significant changes in final body weight with slight, statistically significant increases in body weight gain in males administered 1000 or 2000 mg/kg/day and females administered 2000 mg/kg/day sodium sulfate compared to concurrent controls. No adverse clinical observations were noted during the course of the study. MN-RET frequency and DNA damage were not significantly different from concurrent controls as tested via the MN assay and comet assay, respectively. MN and comet assay results for sodium sulfate are summarized inTable 3 and Table 4, respectively.
For both CGTase and sodium sulfate studies, administration of EMS, the positive control, to male and female mice resulted in a significant increase in MN frequency in peripheral blood and an increase in % tail DNA for liver, stomach, and duodenum when compared to the concurrent controls.
3.4. Results for the 90-day study
3.4.1. Dose formulation and analysis
Formulation analysis for concentration, uniformity, and homogeneity performed monthly were generally greater than theoretical concentrations with minor exceptions involving low or mid-dose formulations (data not shown). These exceptions did not negatively impact the study or affect the ability to establish a NOAEL.
3.4.2. Mortality/moribundity and clinical observations
Two males did not survive to study termination. One in the 250 mg/kg/day CGTase group died immediately following gavage of the 62nd dose administration and one in the 500 mg/kg/day group was euthanized for humane reasons following 87 days of CGTase administration. Macroscopic evaluation attributed these early deaths to gavage error. Tissue weights and clinical pathology data collected from the euthanized rat were not used in statistical analyses. No abnormal cage-side clinical abnormalities attributed to CGTase were observed during the course of the study. Mild corneal crystals in four rats (one male and three females) were observed prior to initiation of dose administration and in one additional rat (control male) a week before study termination.
3.4.3. Body weight and food consumption
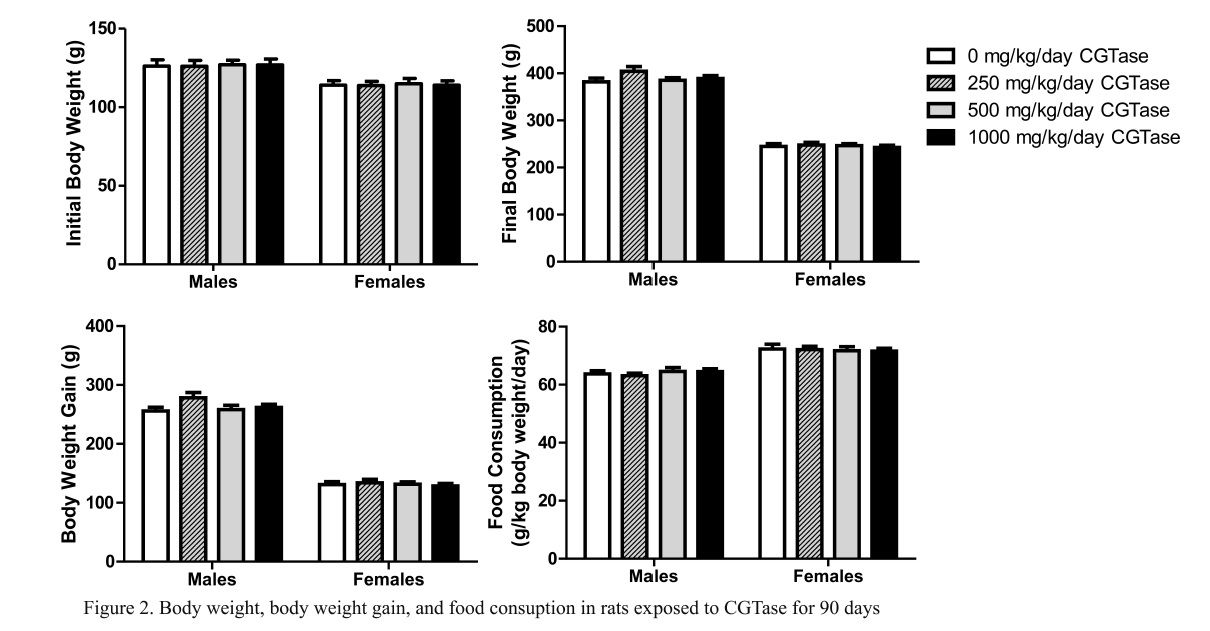
Mean initial and final body weight and body weight gain following 90 consecutive days of CGTase administration are presented in Fig. 2. There were no significant changes in final body weight or body weight gain in rats given CGTase when compared to concurrent controls. Likewise there were no significant changes in mean feed consumption in CGTase treated rats versus concurrent controls (Fig. 2).
3.4.4. Neurotoxicity Screening
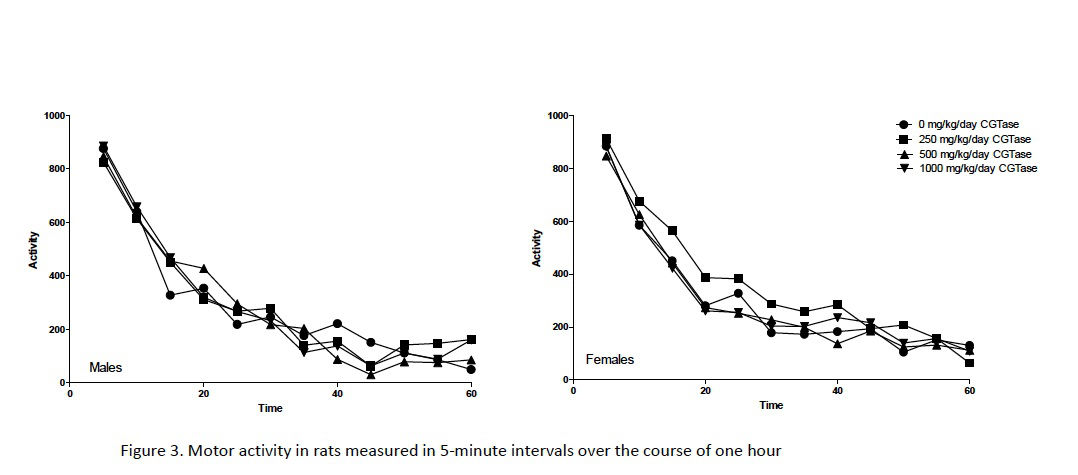
Automated motor activity assessments following 80–82 days of dosing showed no significant changes in rats administered CGTase versus concurrent controls (Fig. 3). Functional observations made blind to treatment (e.g., home cage behavior, handling reactivity, visual and tactile responses, air righting, limb grip strength, hindlimb splay, etc.) identified very few alterations (data not shown). The only statistically significant effect of dose was in landing hindlimb splay in females administered 1000 mg/kg/day CGTase. Hindlimb splay is a measure of a response that requires proprioception and muscle strength. Since no similar changes were seen in any of the other assessments that involve these modalities, such as gait, righting, and extensor thrust, this effect in the 1000 mg/kg/day CGTase female is unlikely to be biologically significant. Furthermore, sciatic nerves were histopathologically normal during subsequent microscopic evaluation. Based on these findings, administration of CGTase at up to 1000 mg/kg/day is unlikely to present a neurotoxicity hazard based on OECD neurotoxicity guidelines (OECD, 1997b).
3.4.5. Urinalysis
Following an 18-hour urine collection, there were no significant pairwise changes in specific gravity, bilirubin, ketones, blood, pH, protein, total volume, glucose, urobilinogen, sodium, and potassium in male or female rats administered CGTase as compared to the concurrent control groups. There was a statistically significant increasing trend in specific gravity and urine potassium levels in female rats. Mean specific gravity values for females were 1.011 ± 0.004, 1.014 ± 0.003, 1.015 ± 0.004, and 1.015 ± 0.004 specific gravity units for control, low-dose, mid-dose, and high-dose rats, respectively. Mean female potassium milliequivalents/L were 35.3 ± 15.1, 43.2 ± 15.1, 50.7 ± 15.7 and 50.0 ± 17.7 for control, low-dose, mid-dose, and high-dose rats, respectively. No other significant dose-dependent trends were measured. The significance of these two trends in female rats is unknown.
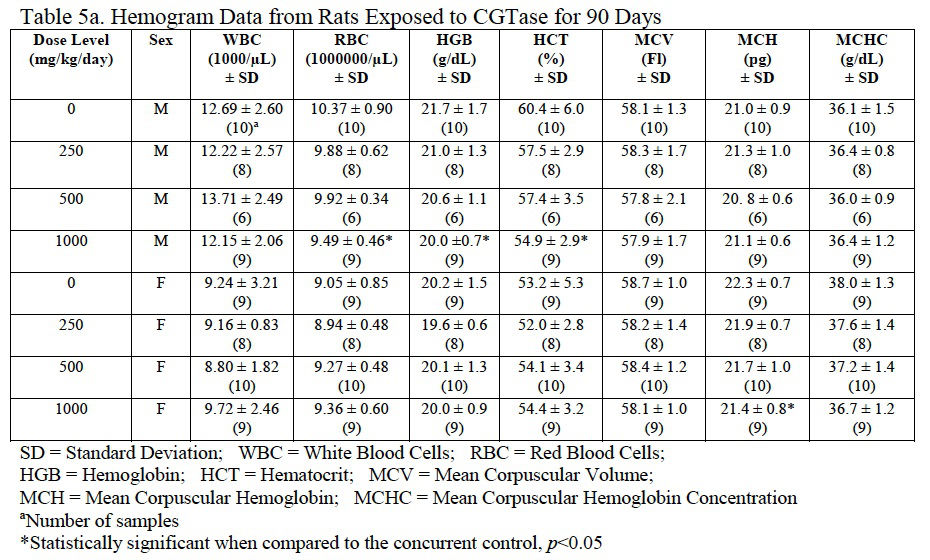
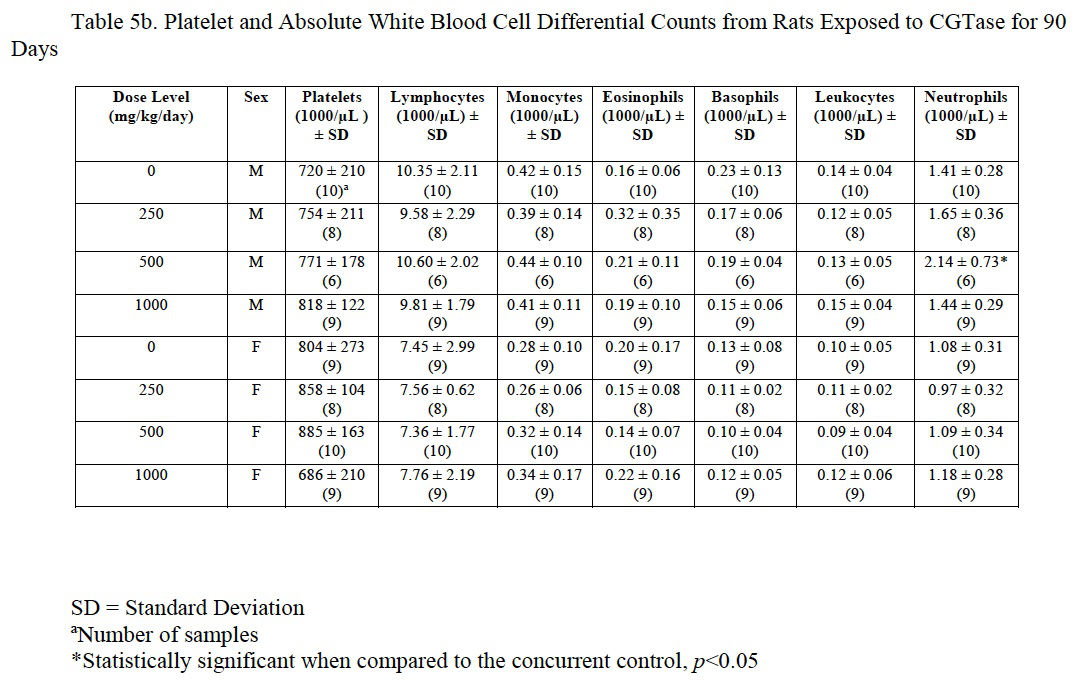
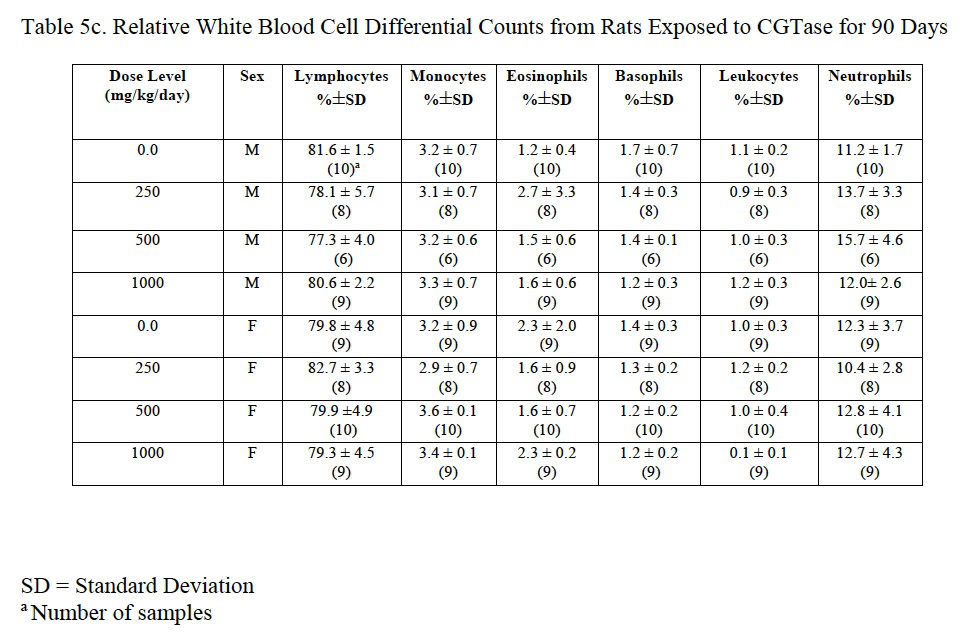
3.4.6. Hematology
Hemogram data are presented in Table 5a, Table 5b and Table 5c. The low platelet count in high-dose females was not statistically different from the control group and was within the laboratory reference range for this rat strain and age. There was a statistically significant decrease in red blood cells (92% of control), hemoglobin (92% of control), and hematocrit (91% of control) in male rats administered 1000 mg/kg/day CGTase as compared to the concurrent controls; there was a significant increase in % neutrophils (139% of controls) and absolute neutrophils (152% of controls) in males administered 500 mg/kg/day CGTase compared to the concurrent control group with a significant corresponding increasing trend. There was a significant decreasing trend in percent lymphocytes in males without any statistically significant associated decreases in any of the male dose groups. MCH (97% of control) was statistically decreased in female rats administered 1000 mg/kg/day CGTase compared to the concurrent control group. Significant changes in hemogram results were within the performing laboratory reference range with the exception of elevated hemoglobin in males that was slightly above the laboratory reference range. There were no microscopic correlates related to the statistically significant hemogram effects.
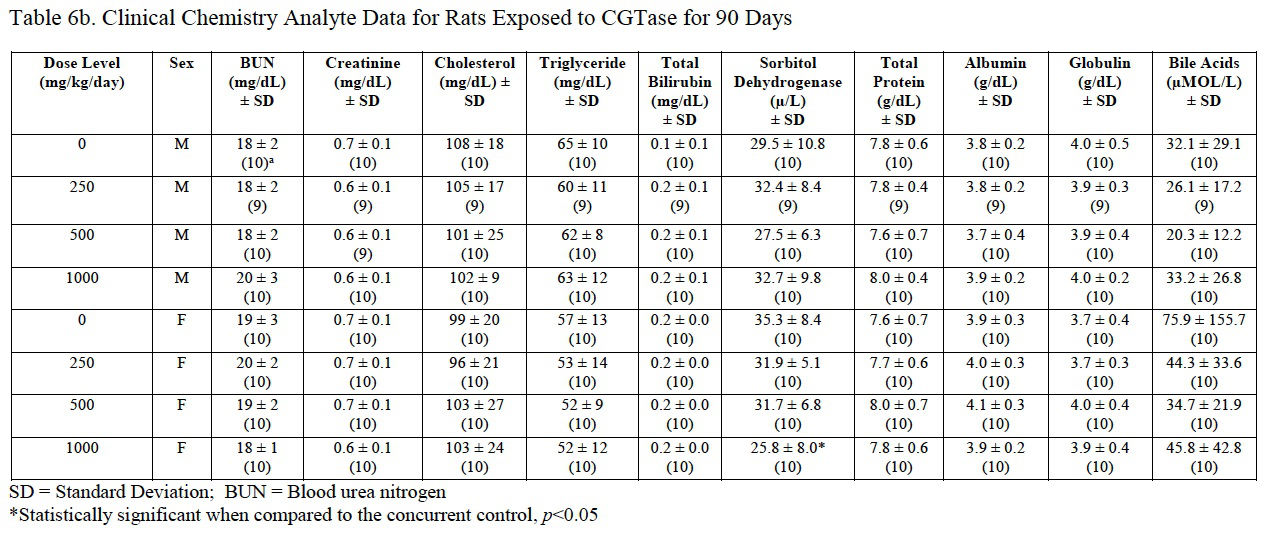
3.4.7. Serum clinical chemistry
Clinical chemistry analyte data are presented in Table 6a and Table 6b. A significant increase in glucose (162% of control) and a significant decrease in sorbitol dehydrogenase (SDH; 73% of control) were measured in female rats administered 1000 mg/kg/day CGTase compared to the concurrent controls. The significantly decreased SDH result was within the performing laboratory reference range whereas the elevated glucose level was above the reference range. The increased glucose concentration may be a result of the administration of the test article containing 86% carbohydrates. There was no effect of CGTase administration on glucose or SDH levels in male rats. There were no histopathological changes that correlate with the observed serum chemistry changes.
3.4.8. Coagulation and hormone analyses
Prothrombin, activated partial thromboplastin times, thyroxine (T4) and thyroid stimulating hormone (TSH) were not significantly changed for either gender compared to concurrent controls (Table 7). Serum triiodothyronine (T3) levels were significantly decreased in male rats administered 1000 mg/kg/day CGTase (77% of control) and significantly increased in female rats administered 500 or 1000 mg/kg/day CGTase (124% and 129%, respectively). Serum T3 levels in female rats exhibited a significant dose-dependent decrease. Serum T3 values for male and female rats were outside the performing laboratory reference range. There were no microscopic findings in the thyroid or other endocrine organs that reflected the changes in serum T3 values.
3.4.9. Macroscopic findings and tissue weights
There were no macroscopic findings at necropsy or changes in tissue weights that were related to CGTase exposure.
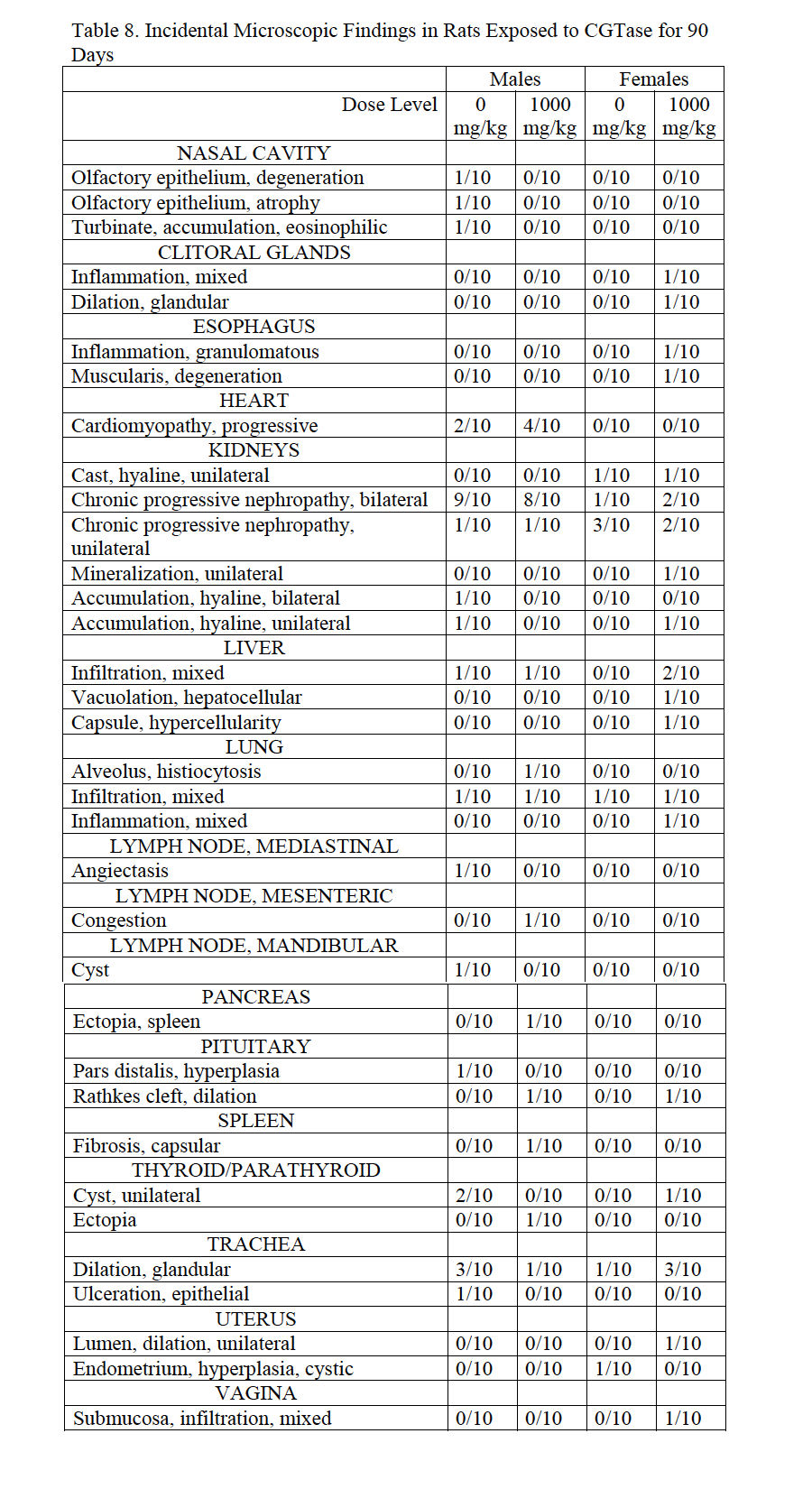
3.4.10. Histopathology
Histopathological findings from high-dose and control male and females reflect common incidental background lesions seen in Sprague Dawley rats of this age with incidences listed in Table 8. Observations that correlate with macroscopic observations include colonic lymphoid hyperplasia (250 mg/kg/day male), decreased secretion in seminal vesicles (500 mg/kg/day male), splenic ectopia in the mesentery (500 mg/kg/day male), lymph node lymphoid hyperplasia and plasmacytosis (500 mg/kg/day male), hepatodiaphragmatic nodule and lymph node congestion (500 mg/kg/day female).
4. Discussion
CGTase, a natural microbial enzyme produced by Bacillus pseudoalcaliphilus DK-1139, can be used to efficiently glycosylate molecules such as isoquercitrin to produce an enzyme modified product for use as a food ingredient. Even though CGTase is heat inactivated and not present in the final product generated from its use, international guidelines call for safety assessment of microbial derived human and animal food products and additives ( EFSA, 2009; EFSA, 2014). Based on results of dose range-finding studies, we used a high limit dose of 2000 and 1000 mg/kg/day in 3-day micronucleus/comet and 90-day toxicity studies, respectively, in accordance with regulatory testing guidelines for non-toxic materials ( OECD, 1998, OECD, 2014a and OECD, 2014b). We conducted a battery of in vitro and in vivo genotoxicity assays and a 90-day repeated dose gavage study in Sprague Dawley rats with no evidence of genotoxicity or systemic toxicity using CGTase from Bacillus pseudoalcaliphilus DK-1139. We also report negative genotoxicity for sodium sulfate that is present at low levels in preparations of CGTase.
Much contemporary research is focused on the genetic underpinnings related to the biological role as well as production and applications of CGTases including use of recombinant DNA technology to modify natural microbial enzymes for specific food processing needs (Qi and Zimmermann, 2005, Olempska-Beer et al., 2006, Leemhuis et al., 2010, Kelly et al., 2009 and Han et al., 2014). We identified one safety evaluation of a CGTase from a recombinant bacterial strain with results similar to our study (Bar et al., 2004). In that study there was no evidence of genotoxicity and the only systemic effects were non-dose-related pulmonary irritation secondary to reflux of the gavaged test material. The authors concluded that their CGTase was safe for the intended use in production of alpha-cyclodextrin.
How much, if any, of the orally administered CGTase was absorbed in the present study is unknown. Negative results of our GLP genotoxicity battery on CGTase and sodium sulfate and lack of adverse toxicity in rats exposed to a limit dose of 1000 mg/kg/day for 90 days support the safe use of CGTase in the production of alpha-glycosyl isoquercitrin.
Acknowledgements
This work was conducted at ILS, Inc. and funded by San-Ei Gen, F.F.I., Inc. Maronpot Consulting LLC is a consultant for San-Ei Gen, F.F.I., Inc. and was responsible for writing the manuscript. ILS, Inc. was responsible for the study design, the collection, analysis, and interpretation of data, and the writing of the final study report. The decision to submit the paper for publication was made by San-Ei Gen, F.F.I., Inc. The authors are indebted to Kim Shepard, John Winters, Katie Rechsteiner, Teresa Mascenik, Colleen Lentz, Anthony Monroe, and Rameeza Mahmood for in vitro technical assistance. The authors also acknowledge the contributions of members of the Investigative Toxicology and Histology Departments at ILS, Inc., including Eileen Phillips and John Pope, who provided animal care, dosing, and necropsy services, and Kaye Cummings and Jeanne deWard for quality assurance review of the data.
References
Alera Labs, 2014
Alera Labs
Stability Studies for Cyclodextrin Glucanotransferase (CGTase) Dosing Solutions
Stability Summary Report ILS08-003.00, Alera Labs, LLC (2014)
Ames et al., 1975
B.N. Ames, J. McCann, E. Yamasaki
Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test
Mutat Res, 31 (1975), pp. 347–364
Avlasevich et al., 2006
S.L. Avlasevich, S.M. Bryce, S.E. Cairns, S.D. Dertinger
In vitro micronucleus scoring by flow cytometry: differential staining of micronuclei versus apoptotic and necrotic chromatin enhances assay reliability
Environ Mol Mutagen, 47 (2006), pp. 56–66
Bar et al., 2004
A. Bar, C.A. Krul, D. Jonker, N. de Vogel
Safety evaluation of an alpha-cyclodextrin glycosyltranferase preparation
Regul Toxicol Pharmacol, 39 (Suppl. 1) (2004), pp. S47–56
Bryce et al., 2007
S.M. Bryce, J.C. Bemis, S.L. Avlasevich, S.D. Dertinger
In vitro micronucleus assay scored by flow cytometry provides a comprehensive evaluation of cytogenetic damage and cytotoxicity
Mutat Res, 630 (2007), pp. 78–91
EFSA, 2009
EFSA
Guidance of the Scientific Panel of Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) on the Submission of a Dossier on Food Enzymes for Safety Evaluation by the Scientific Panel of Food Contact Material, Enzymes, Flavourings and Processing Aids
The EFSA Journal, 1305 (2009), pp. 1–26
EFSA, 2014
EFSA (2014). Technical Report of EFSA. Explanatory Note for the Guidance of the Scientific Panel of Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) on the Submission of a Dossier on Food Enzymes. EFSA Supporting Publication: EN-579.
Han et al., 2014
R. Han, J. Li, H.D. Shin, R.R. Chen, G. Du, L. Liu, J. Chen
Recent advances in discovery heterologous expression, and molecular engineering of cyclodextrin glycosyltransferase for versatile applications
Biotechnol Adv, 32 (2014), pp. 415–428
Hedges, 1998
A.R. Hedges
Industrial Applications of Cyclodextrins
Chem Rev, 98 (1998), pp. 2035–2044
Kelly et al., 2009
R.M. Kelly, L. Dijkhuizen, H. Leemhuis
The evolution of cyclodextrin glucanotransferase product specificity
Appl Microbiol Biotechnol, 84 (2009), pp. 119–133
Leemhuis et al., 2010
H. Leemhuis, R.M. Kelly, L. Dijkhuizen
Engineering of cyclodextrin glucanotransferases and the impact for biotechnological applications
Appl Microbiol Biotechnol, 85 (2010), pp. 823–835
Maron and Ames, 1983
D.M. Maron, B.N. Ames
Revised methods for the Salmonella mutagenicity test
Mutat Res, 113 (1983), pp. 173–215
Mortelmans and Zeiger, 2000
K. Mortelmans, E. Zeiger
The Ames Salmonella/microsome mutagenicity assay
Mutat Res, 455 (2000), pp. 29–60
OECD, 1997a
OECD, OECD Guideline for the Testing of Chemicals: Bacterial Reverse Mutation Test. TG 471, (1997a).
OECD, 1997b
OECD, OECD Guideline for the Testing of Chemicals: Neurotoxicity Study in Rodents. TG 424. (1997b).
OECD, 1998
OECD, OECD Guideline for the Testing of Chemicals: Repeated Dose 90-Day Oral Toxicity Study in Rodents. TG 408, (1998).
OECD, 2014a
OECD, OECD Guideline for the Testing of Chemicals: In Vitro Mammalian Cell Micronucleus Test. TG 487, (2014a).
OECD, 2014b
OECD, OECD Guideline for the Testing of Chemicals: Mammalian Erythrocyte Micronucleus Test. TG 474, (2014b).
OECD, 2014c
OECD, OECD Guideline for the Testing of Chemicals: In Vivo Mammalian Comet Assay. TG 489, (2014c).
Olempska-Beer et al., 2006
Z.S. Olempska-Beer, R.I. Merker, M.D. Ditto, M.J. DiNovi
Food-processing enzymes from recombinant microorganisms–a review
Regul Toxicol Pharmacol, 45 (2006), pp. 144–158
Qi and Zimmermann, 2005
Q. Qi, W. Zimmermann
Cyclodextrin glucanotransferase: from gene to applications
Appl Microbiol Biotechnol, 66 (2005), pp. 475–485
Recio et al., 2012
L. Recio, G.E. Kissling, C.A. Hobbs, K.L. Witt
Comparison of Comet assay dose-response for ethyl methanesulfonate using freshly prepared versus cryopreserved tissues
Environ Mol Mutagen, 53 (2012), pp. 101–113
Thompson, 1997
D.O. Thompson
Cyclodextrins–enabling excipients: their present and future use in pharmaceuticals
Crit Rev Ther Drug Carrier Syst, 14 (1997), pp. 1–104
Witt et al., 2008
K.L. Witt, E. Livanos, G.E. Kissling, D.K. Torous, W. Caspary, R.R. Tice, L. Recio
Comparison of flow cytometry- and microscopy-based methods for measuring micronucleated reticulocyte frequencies in rodents treated with nongenotoxic and genotoxic chemicals
Mutat Res, 649 (2008), pp. 101–113
Note to users:
Accepted manuscripts are Articles in Press that have been peer reviewed and accepted for publication by the Editorial Board of this publication. They have not yet been copy edited and/or formatted in the publication house style, and may not yet have the full ScienceDirect functionality, e.g., supplementary files may still need to be added, links to references may not resolve yet etc. The text could still change before final publication.
Although accepted manuscripts do not have all bibliographic details available yet, they can already be cited using the year of online publication and the DOI, as follows: author(s), article title, Publication (year), DOI. Please consult the journal’s reference style for the exact appearance of these elements, abbreviation of journal names and use of punctuation.
When the final article is assigned to volumes/issues of the Publication, the Article in Press version will be removed and the final version will appear in the associated published volumes/issues of the Publication. The date the article was first made available online will be carried over.