Environmental pollutants, chemicals, and drugs have an impact on children’s immune system development. Mexico City (MC) children exposed to significant concentrations of air pollutants exhibit chronic respiratory inflammation, systemic inflammation, neuroinflammation, and cognitive deficits. We tested the hypothesis that exposure to severe air pollution plays a role in the immune responses of asymptomatic, apparently healthy children. Blood measurements for markers of immune function, inflammatory mediators, and molecules interacting with the lipopolysaccharide recognition complex were obtained from two cohorts of matched children (aged 9.7 ± 1.2 years) from southwest Mexico City (SWMC) (n = 66) and from a control city (n = 93) with criteria pollutant levels below current standards. MC children exhibited significant decreases in the numbers of natural killer cells (p = .003) and increased numbers of mCD14+ monocytes (p < .001) and CD8+ cells (p = .02). Lower concentrations of interferon γ (p= .009) and granulocyte–macrophage colony-stimulating factor (p < .001), an endotoxin tolerance-like state, systemic inflammation, and an anti-inflammatory response were also present in the highly exposed children. C-reactive protein and the prostaglandin E metabolite levels were positively correlated with twenty-four- and forty-eight-hour cumulative concentrations of PM2.5. Exposure to urban air pollution is associated with immunodysregulation and systemic inflammation in children and is a major health threat.
Introduction
Environmental pollutants, chemicals, and drugs have a negative impact on the developing immune system in children (Landrigan et al. 2004; Richter-Reichhelm et al. 2002). Rodent immunotoxicity data have shown that suppression of immune responses in rodents is predictive of a similar response in humans and that there is a relationship between immune suppression following developmental exposure to toxicants and enhanced risk of infectious or neoplastic disease in humans (Selgrade 2007). Exposure to environmental tobacco smoke significantly alters immune effectors and may contribute to an increased incidence of allergic and infectious diseases in exposed children (Landrigan 2004; Landrigan et al. 2004; Tebow et al. 2008; Wang et al. 2007). Epidemiological data also indicate that children of mothers who smoke during pregnancy or parents occupationally exposed to particulate matter have a greater risk of developing tumors of the nervous system, leukemias, and lymphomas (Filippini et al. 2000; Magnani et al. 1990; Selgrade 2007). Epidemiological studies strongly suggest that children residing in southwest Mexico City (SWMC) have an increased prevalence and frequency of leukemias, brain tumors, and lymphomas compared to children residing in north Mexico City (MC) (Fajardo-Gutiérrez et al. 1997). In the last two decades, pediatricians from the Instituto Nacional de Pediatría have observed an increased number of previously healthy children with severe complications from varicella (Cárdenas-Monteverde 2006). These children have no known risk factors for the development of severe complications of this common, usually benign, and self-limiting infectious disease.
Air quality in MC has been recognized among the worst in the world (Bravo-Alvarez and Torres-Jardón 2002). Since pollution levels in MC have been sustained or worsened in the past twenty years, exposures of today’s children and teenagers are truly life long (Bravo-Alvarez and Torres-Jardón 2002; Vega et al. 2004). MC children are exposed all year long, several hours per day, to a significant burden of air pollutants, including concentrations above the current U.S. standards for ozone (O3) and particulate matter < 10 μm and < 2.5 μm in diameter (PM10, PM2.5), as well as lipopolysaccharides associated with PM (PM-LPS) (Bonner et al. 1998; Bravo-Alvarez and Torres-Jardón 2002; Villarreal-Calderón et al. 2002).
On average, SWMC middle school children engage in play 19.6 hours/week and participate in outdoor physical activities throughout the year in the late morning and afternoon, when the pollutant levels are at their maximum (Villarreal-Calderón et al. 2002). These children are exposed an average of 3 hours per day to ozone above 0.08 ppm, and their annual PM10 and PM2.5 exposures average 78 and 25 μg/m3, concentrations well above their respective annual standard of 50 and 15 μg/m3 (Bravo-Alvarez and Torres-Jardón 2002; Calderón-Garcidueñas, Franco-Lira et al. 2007;Calderón-Garcidueñas, Vincent et al. 2007; Vega et al. 2004).
It has been suggested that immune system status may be influenced by air pollutants (American Thoracic Society 1996), a setting clearly present in asthmatic children in whom alterations in immunoinflammatory biomarkers are associated with ambient PM (Svendsen et al. 2007). In a cross-sectional study from seventeen Central European cities, immune markers in relation to exposure to air pollutants were examined in 366 children at ages nine to eleven years (Leonardi et al. 2000). The study showed an increase in the numbers of CD4+, CD8+, and NK cells, and IgG concentrations with increasing levels of PM2.5. The annual average of PM2.5 across the seventeen cities ranged from 29 to 67 μg/m3 (Leonardi et al. 2000).
In this study we tested the hypothesis that SWMC children will have alterations in their immune system parameters, along with systemic inflammation, based on their sustained lifelong exposures to significant concentrations of air pollutants, including PM2.5. The primary objective of this study was to measure specific markers of innate and adaptive immunity in children from two urban areas with sustained, significantly different levels of air pollution. A second objective was to determine whether these children have alterations in immunoregulatory cytokines, essential for both innate and adaptive immune responses. A third objective was to confirm the presence of systemic inflammation described in previous cohorts of MC children (Calderón-Garcidueñas et al. 2003; Calderón-Garcidueñas, Villarreal-Calderón et al. 2008), and a fourth objective was to investigate if the key parameters of interest were correlated with pollutant exposure levels. This study will add to our understanding of children’s immunological responses to sustained exposures to air pollutants.
Materials and Methods
Study Areas
The areas selected for this study were SWMC and Polotitlán. SWMC has had concentrations of O3 and PM10 above the U.S. National Ambient Air Quality Standard (NAAQS) for the past twenty years (Bravo-Alvarez and Torres-Jardón 2002; Vega et al. 2004). SWMC PM2.5 concentrations have been above the annual NAAQS ever since this pollutant criterion started to be monitored in 2003. The selected area has significant sources of environmental endotoxins, including open sewer channels and daily outdoor deposits of thousands of pounds of animal and human fecal material (Estrada-García et al. 2002). SWMC exhibits the highest endotoxin concentrations in the city (Osornio-Vargas et al. 2003). The selection of children from SWMC was based on three factors: (1) the SW location of the base children’s hospital; (2) children living and attending school close to the hospital; and (3) the location of the two closest atmospheric monitoring stations, Pedregal and Coyoacán. The pollutants that consistently exceeded their respective standard in the past four years were O3, PM10, and PM2.5; thus, for these pollutants we estimated the cumulative exposure levels for each child for one, two, and seven days prior to the peripheral blood sampling.
Polotitlán is located 114 km northwest of MC at 2380 m above sea level, and its selection as a control city was based on five key factors: (1) concentrations for all major air pollutants below the NAAQS; (2) access to a population of healthy children; (3) previous clinical studies with the Polotitlán cohort that indicated that children had no evidence of air pollution–related health issues (Calderón-Garcidueñas, Vincent et al. 2007; Calderón-Garcidueñas, Villareal-Calderón et al. 2008, Calderón-Garcidueñas, Mora-Tiscareño et al. 2008); (4) an altitude above sea level similar to that of MC; and (5) relative proximity to MC to facilitate the follow-up of the control cohort.
Study Population
This prospective protocol was approved by the Review Boards for Human Studies Committee at the Instituto Nacional de Pediatría. Written consent was obtained from the parents and oral consent from the children. The inclusion criteria were: negative smoking history and environmental tobacco exposure documented by negative urinary cotinine; lifelong residency in MC or in the control city; residency within five miles of the monitoring stations; full-term babies; no known exposures to local sources of endotoxins; no indoor pets; unremarkable clinical histories, including negative history of hospitalizations for respiratory illnesses, personal and familial histories of atopic diseases, febrile episodes, or vaccinations in the previous three months; and in the case of control children, negative history of frequent travels outside MC or to a large city. Ninety-three control and sixty-six MC children had complete physical exams by our pediatricians, and fasting blood was drawn between 7 and 9 AM. Both cohorts were examined between November 11, 2004, and January 6, 2005.
Peripheral Blood Analyses
Blood samples were taken for the flow cytometric studies, complete blood count (CBC) with differential, and serum for metabolic panel and the ELISAs. LBP, soluble CD14, IL-10, CRP, IFN-γ, GM-CSF, lactoferrin, and HSP60 were measured by a commercially available ELISA from Cell Sciences (Canton, MA, USA), Calbiochem (Darmstadt, Germany), R&D Systems (Minneapolis, MN, USA), and Stressgen (Victoria, Canada). Prostaglandin E metabolite and corticosterone were measured with a competitive enzyme immunoassay from Cayman Chemical (Ann Arbor, MI, USA). Minimum detection concentrations were LBP, 1 ng/mL; sCD14, 6 ng/mL; IL10, 3 pg/mL; IFN-γ, 0.1 pg/mL; GM-CSF, 0.40 pg/mL; CRP, 0.010 ng/mL; lactoferrin, 1 ng/mL; HSP60, 3 ng/mL; corticosterone, 24 pg/mL; and prostaglandin E metabolite, 2 pg/mL. Iron and total iron-binding capacity were measured by the SYNCHRON Beckman Coulter system using acetate buffer, and the hydroxylamine hydrochloride method. Flow cytometry and immunophenotyping multiparameter analysis used in this study have been previously described in detail (Knapp et al. 1994). For SNP genotyping, the leukocyte buffy coat was used for the extraction of genomic DNA by standard methods. Asp299Gly genotype was determined using an allelic discrimination assay protocol according to Applied Biosystems (Foster City, CA, USA).
Rationale for Selection of Measured Blood Parameters
We measured CD56+/CD3− NK cells to characterize the degree of impairment of the innate immune response, and human leukocyte antigen-DR (CD3+/HLA-DR+) because it is essential for the presentation of peptides derived from ingested microbes to CD4-positive T cells to initiate a specific immune response (Cheadle 1993; Orange 2006;Orange 2008). A panel of lymphocyte subsets included: CD3+ T-lymphocytes, CD3−CD19+ B-lymphocytes, with subsets of CD3+ cells including CD4+, CD8+, the ratio of CD4/CD8, and CD4, CD14 lymphocytes expressing CD69. IFN-γ was selected as a representative of an immunoregulatory cytokine essential for both innate and adaptive immune responses, and critical for successful clearance of intracellular pathogens and in-host defense against malignant transformation (Dunn et al. 2004; Yu et al. 2006). Granulocyte–macrophage colony-stimulating factor (GM-CSF) is a 22-kDa glycoprotein cytokine secreted by mononuclear leukocytes that enhances the capability of monocytes and macrophages to phagocytose invading pathogens (Mellstedt et al. 1999). Lactoferrin is an iron-binding protein that bridges innate and adaptive immune function by regulating target cell responses (Kruzel et al. 2007).
To define the degree of systemic inflammation, we measured inflammatory mediators—C-reactive protein, prostaglandin E metabolite, and heat shock protein 60—and IL-10 was the selected Th2 anti-inflammatory cytokine. Given that controlled ozone exposures are associated with upregulation of mCD14 on airway macrophages and monocytes, and that a synergistic action on the mCD14 effect has been suggested between PM-LPS and ozone (Alexis et al. 2004), we selected mCD14, sCD14, and LPS binding protein (LBP) (Bonner et al. 1998; Fenton and Golenbock 1998) to characterize the LPS recognition complex components. LPS forms a complex with an acute-phase protein called LBP responsible for the binding and transport of LPS in the circulation (Fenton and Golenbock 1998). A major response to LPS is mediated by its interaction with CD14, a 55kDa myeloid differentiation antigen that allows endotoxin to interact with the toll-like receptor 4 (TLR4) (Fenton and Golenbock 1998). Finally, TLR4 specifically recognizes LPS and is part of the endotoxin signaling receptor complex that initiates proinflammatory signaling. Since missense mutations such as Asp299Gly are associated with a blunted response to inhaled LPS, we determined the allelic frequencies of Asp299Gly TLR4 polymorphism in both cohorts and included only children fully capable of responding to LPS (Garantziotis et al. 2008).
Statistical Analysis
The primary variables of interest were the numbers of lymphocyte subtypes including CD8+, CD56+/CD3− NK, HLA-DR+, IFN-γ, and the inflammatory mediators. We analyzed the cumulative values previous to the blood sampling for each MC child at twenty-four hours, forty-eight hours, and seven days for PM2.5, PM10, O3, and NO2 and established correlations with the variables of interest. Two independent sample tests and the Pearson’s correlation test were applied. We considered a two-sided type I error rate of 0.05 to be significant when comparing differences between group means. Data are expressed as means±SD. All the statistical computations were performed with the use of Stata 8.3 software (Stata Corp., College Station, TX, USA) or GraphPad Prism v 3.3 (GraphPad Software Inc., San Diego, CA, USA).
Results
Air Pollution Levels
MC children in this study have been exposed to significant concentrations of O3 and PM for their entire lives (Calderón-Garcidueñas, Vincent et al. 2007; Calderón-Garcidueñas, Villareal-Calderón et al. 2008; Calderón-Garcidueñas, Solt et al. 2008; Calderón-Garcidueñas, Mora-Tiscareño et al. 2008). The climatic conditions in MC are relatively stable, thus pollutant concentrations are consistent year after year. In MC the higher eight-hour O3 concentrations coincide with the times children are outdoors during school recess and physical education periods and when they play outdoors at home (Villarreal-Calderón et al. 2002). Because of the existing high correlation between secondary organic aerosols and photochemical processes, PM10 concentrations in MC also tend to peak during the mid-afternoon hours that coincide with children’s outdoor activities. Children in SWMC are exposed to a yearly PM2.5 average of 25 μg/m3(Annual Standard below 15 μg/m3). During the study period, PM2.5 concentrations in SWMC were 35.89 ± 0.93 μm/m3. In the control city (Polotitlán), because of the combination of the relatively few contributing emission sources from industry and cars and the good ventilation conditions by the regional wind, historically O3, PM10, SO2, NO2, and CO levels are below the current U.S. standards (Calderón-Garcidueñas, Vincent et al. 2007; Calderón-Garcidueñas, Villareal-Calderón et al. 2008; Calderón-Garcidueñas, Solt et al. 2008).
Demographics and Physical Exams
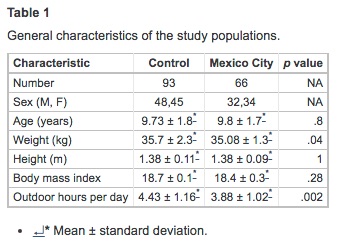
All participant children were from middle-class families who lived in single-family houses. No occupational toxic exposures were reported by parents or close relatives. Children slept in bedrooms with no carpeting and had open windows for ventilation. All households had kitchens separated from the living and sleeping areas and used gas for cooking. On physical exam, the vital signs and the physical examination performed by the pediatrician were unremarkable in all participant children. Table 1 summarizes the characteristics of the matched population. There were no differences in the age, gender distribution, and height. MC children were slightly thinner than control children (p = .04), although the body mass index showed no differences. No overweight or obese children were included. Control children spent more time outdoors that MC children (p = .002).
Laboratory Findings
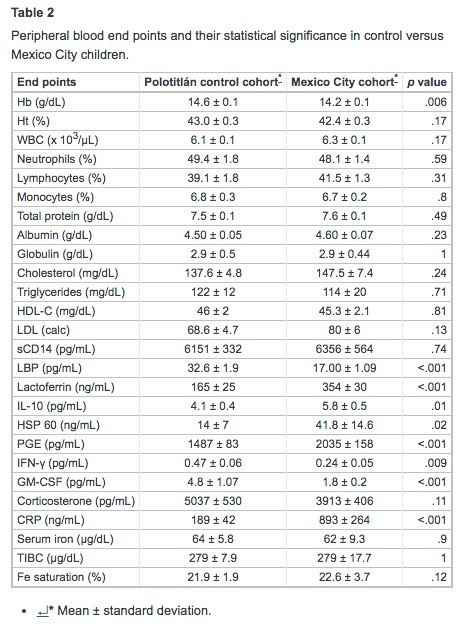
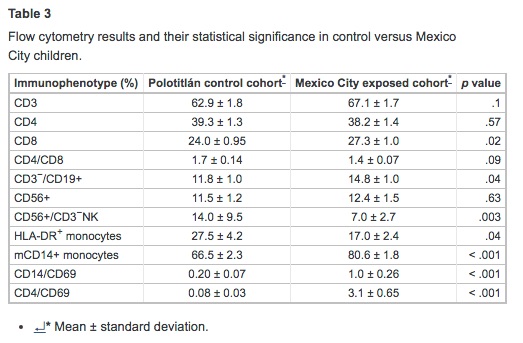
Tables 2 and 3 summarize the laboratory findings and their statistical significance. There were no significant differences between cohorts in Ht, WBC differential counts, total protein, albumin, globulins, lipids, sCD14, and corticosterone values. MC children had lower concentrations of Hb; however, serum iron, unsaturated iron-binding capacity, and percent saturation were within normal limits in both cohorts. SWMC children had significantly higher concentrations of PGE and CRP (p = .001), LF (p < .001), IL-10 (p = .01), and HSP60 (p = .02). SWMC children exhibited an increase in monocytic expression of mCD14 (p < .001), whereas LBP was significantly decreased (p < .001). The LBP/sCD14 ratio was significantly lower in the MC cohort (p < .001). MC children exhibited significant decreases in serum concentrations of IFN-γ (p = .009) and GM-CSF (p < .001). There were significant correlations between CRP and LBP (r2 = 0.51, p< .001) and age and LF (r2 = −0.32, p = .003). Flow cytometric studies showed MC children to have a significant decrease in the numbers of NK (p = .003) and HLA-DR+ cells (p = .04), and increased percentages of CD8+ (p = .02), B-lymphocytes (p = .04), and mCD14+monocytes (p < .001) (Table 3).
Correlation of C-reactive Protein and Prostaglandin-E Metabolite Concentrations and PM2.5 and PM10
We examined the cumulative exposure concentrations of PM2.5, PM10, and O3 for each MC child over the one, two, and seven days preceding the measurement of all end points. CRP and PGE metabolite gave significant positive correlations with PM2.5 and PM10. PGE gave a correlation with cumulative PM2.5 exposure at forty-eight hours (r = 0.34, p = .02) and with twenty-four-hour PM10 (r = 0.35, p = .03), and CRP gave a positive correlation with cumulative PM2.5 exposure at twenty-four hours (r = 0.24, p = .02) and forty-eight hours (r = 0.26, p =.01). The average cumulative concentrations of PM2.5 for SWMC children over the twenty-four-hour and forty-eight-hour periods preceding the measurement of CRP and PGE levels were 362.8 ± 113.4 and 760.8 ± 120.1 μg, respectively.
Discussion
A major concern of MC pediatricians, infectiologists, and epidemiologists is the potential impact of the environment on the immune responses of exposed children (Cárdenas-Monteverde 2006; Fajardo-Gutiérrez et al. 1997). The major adverse responses to the immune system injury include direct immunotoxicity (immunosupression or immunostimulation), hypersensitivity, and autoimmunity (Descotes et al. 2000).
This study allowed us to explore a crucial question: what is the impact of a highly polluted environment on the immune and systemic inflammatory responses in clinically healthy children? The results suggest that lifelong exposure to a highly polluted environment including high concentrations of PM2.5 is associated with a significant decrease in the numbers of natural killer and HLA-DR+ cells, lower concentrations of IFN-γ and GM-CSF, and activated CD4 and CD14 cells bearing markers of recent stimulation (CD69). Similar to the results by Leonardi et al. (2000) in the cross-sectional survey of children in seventeen cities of Central Europe, we also observed increases in peripheral CD8+ and B lymphocytes.
At the crux of the immunodysregulation and the brisk anti-inflammatory response, as evidenced by increased IL-10, is the sustained state of systemic inflammation in SWMC children. Given that these children are continuously exposed to air pollutants, their upper and lower respiratory inflammatory processes and their systemic responses are relentless, a crucial point to understand the counter-regulatory loops that are taking place in these children (Calderón-Garcidueñas et al. 2003, Calderón-Garcidueñas, Vincent et al. 2007, Calderón-Garcidueñas, Mora-Tiscareño et al. 2008). The sustained inflammatory process alters the immune status of circulating leukocytes, as illustrated by the reduced numbers of NK cells seen in septic processes (Cavaillon and Adib-Conquy 2007). NK cell functions are essential in the control of numerous infections, including herpes virus and cancer, and NK cell proliferation, trafficking, and cytotoxicity can be either activated or inhibited by a variety of signal transduction surface receptors and by cytokines (Biron et al. 1999; Cavaillon and Adib-Conquy 2007; Orange 2006;Orange 2008; Wu and Lanier 2003). SWMC children exhibit a NK cell deficiency based on a reduction in the number of NK cells, and thus low concentrations of IFN-γ are expected (Orange 2006). Pro- and anti-inflammatory cytokine signaling reciprocally antagonize regulation of NK cell IFN-γ production (Yu et al. 2006). IFN-γ is strongly produced only by activated T cells and NK cells (Maher et al. 2007). IFN types, including IFN-γ, signaling primarily through the JAK/STAT pathway, lead to the transcription of genes important in the antiproliferative, antiviral, immunomodulatory, and/or apoptotic responses necessary for modulation of an effective innate immune response against viruses and intracellular pathogens (Maher et al. 2007). Low concentrations of IFN-γ could result in a higher susceptibility to infections and malignant processes (Maher et al. 2007; Yu et al. 2006). One key role of IFNs in promoting protective immune responses is their ability to regulate the expression of MHC proteins (Maher et al. 2007).
SWMC children exhibited a significant decrease in monocytic HLA-DR+ expression, a glycosylated cell surface transmembrane protein expressed on antigen-presenting cells (Perry et al. 2004). Impaired monocyte function has been described in sepsis and has been characterized by inadequate respiratory burst, activation, antigen presentation, and lowered expression of HLA-DR (Cheadle 1993; Cavaillon and Adib-Conquy 2007;Perry et al. 2004). The infusion of a low dose of endotoxin into healthy volunteers results in complex changes in HLA-DR, production of pro- and anti-inflammatory cytokines, and activation of coagulation (Mant et al. 2008). GM-CSF, also downregulated in SWMC children, functions as a growth and differentiation factor for immature phagocytic cells, induces IL-12 production, and polarizes T lymphocytes toward a type 1 proinflammatory response, thereby increasing the capability of monocytes and macrophages to phagocytose invading pathogens (Eksioglu et al. 2007;Mellstedt et al. 1999). Type 2 cytokines are downregulated by GM-CSF, and proliferation of allogeneic T cells is increased (Eksioglu et al. 2007). The upregulation of CRP and PGE was expected, given the involvement of the respiratory tract in exposed children (Calderón-Garcidueñas et al. 2003). CRP, a pattern-recognition molecule that increases within hours after tissue injury or infection, contributes to host defense, and it is part of the innate immune response (Black et al. 2004). The pleiotropic effects of CRP are reflected in anti-inflammatory effects, including increasing the release of IL-10 and decreasing IFN-γ synthesis (Szalai et al. 2002).
We found modest correlations in our SWMC cohort between CRP and PM2.5cumulative exposure at twenty-four hours (r = 0.24) and forty-eight hours (r = 0.26), similar to the results described in young adults and children from Japan and Taiwan (Chuang et al. 2007; Shima 2007). Our results of a significant upregulation of prostaglandin E metabolite and the positive correlations with cumulative PM2.5 and PM10 are a very ominous finding given the role of prostaglandins in chronic inflammation and human carcinogenesis (Federico et al. 2007; Wang and Dubois 2006). The long-term carcinogenic effects of sustained elevated concentrations of prostaglandins and cycloxygenase-2–derived PGE2 in children are not known; however, we know that both prostaglandins and inflammatory cytokines link inflammation to cancer via oxidative/nitrosative stress (Federico et al. 2007).
Recognition of LPS involves the cooperation between LBP, the membrane-bound or the soluble forms of CD14, and TLR4 (Heumann and Roger 2002). Children selected for this study did not carry the Asp299Gly TLR4 polymorphism, and thus included children are likely capable of responding to LPS. SWMC children had increased monocytic mCD14 in keeping with the work of Alexis et al. (2004) associating ozone exposures with upregulation of mCD14 on airway macrophages and monocytes, and a synergistic action on the mCD14 effect between PM-LPS and ozone. Monocyte membrane CD14 is the key membranous receptor involved in LPS binding, and its upregulation represents the early step in cell activation by LPS involving the innate immune initial host response to Gram-negative bacterial infections (Heumann and Roger 2002). The mCD14 upregulation observed in exposed MC children likely relates to its two major functions: a functional receptor for LPS and a scavenger for LPS (Heumann and Roger 2002) in a population historically exposed to significant concentrations of endotoxins associated with PM (Bonner et al. 1998; Osornio-Vargas et al. 2003). LBP properties are likely concentration dependent, and although serum levels increase in the setting of sepsis, rendering the host more sensitive to LPS, at low concentrations LBP may potentiate macrophage activation by LPS (Fan et al. 2002).
The lower LBP concentrations observed in SWMC children could be explained by at least three potential mechanisms: (1) the displacement of LBP from the binding lipid A site by LF and HSP60, both displaying a tightly bound affinity for the same site; (2) the mechanism of extravasation of LBP in bronchoalveolar lavage fluid; and (3) by LBP downregulation that would allow for cellular activation by the major chemotypes of LPS (Dubin et al. 1996; Habich 2005; Hamann et al. 2005). LBP can be displaced from the binding lipid A site by two known LPS-binding proteins, LF and HSP 60, both shown here to be significantly higher in MC children (Hamann et al. 2005). Plasma LF concentrations are very low in healthy people, and blood LF is considered to be neutrophil derived and an indicator of neutrophil turnover (Krusel et al. 2007). Interestingly, the protective effect of LF in E coli–induced bacteremia in mice is associated with an increase turnover of neutrophils, both in the bone marrow and in peripheral blood, with a significant increase in the percentage of neutrophils, both mature forms and bands (Zimecki et al. 2004).
Review of the CBC showed no differences in the percentage of PMNs, the total numbers of neutrophils, or increased numbers of bands. In the context of an alteration in plasma proteins as a response to infections, high-density lipoproteins are capable of LPS sequestration (Levels et al. 2005). This sequestration attenuates the host response to infection but produces a dyslipidemia that severely compromises the host defense mechanisms (Levels et al. 2005). LBP transfers LPS from predominantly high-density lipoproteins (HDL) to low-density lipoproteins (LDL) in a time- and dose-dependent manner (Levels et al. 2005). Levels et al. postulated that these HDL-remodeling changes induced by LBP-mediated LPS transfer may contribute to the plasma lipoprotein dyslipidemia characteristic of the acute phase response to infection. Lipid profiles showed no differences between controls and exposed children, and no evidence of dyslipidemia. The potential clinical importance of low LBP in MC children is illustrated in LBP-deficient mice having impaired resistance to lung infections by K. pneumoniae; additionally, the lower LBP/sCD14 ratio could have an impact on the risk of atherosclerosis, as described in subjects with chronic subacute infections (Fan et al. 2002; Tapping and Tobias 2000).
Early LPS recognition is crucial for the host to build up an immune reaction against bacteria (Hamann et al. 2005). Mechanisms that lessen an immune reaction initiated by LPS include the induction of cellular and systemic states of LPS tolerance; cellular internalization and endolysosomal deacylation of the major endotoxic LPS forms; and the neutralizing transfer of LPS to HDL, followed by intestinal excretion via the liver–bile duct pathway (West and Heagy 2002; Ziegler-Heitbrock 1995). The development of an endotoxin tolerance state is an important issue to consider in MC children. Reduced monocyte HLA-DR expression is regarded as a diagnostic marker of a temporary immunodeficiency in endotoxin tolerance (Muehlstedt et al. 2002; Wolk et al. 2003). Wolk et al. and Muehlstedt et al. showed that endotoxin priming produces a decrease of monocyte intracellular MHC Class II, along with a reduced export of MHC Class II α β complexes to the cell surface, and that IL-10 produces a similar effect. In SWMC children, the significant increase of IL-10 could certainly contribute to the HLA-DR down regulation (Calderón-Garcidueñas et al. 2003).
Acquired tolerance induction is not restricted to immunostimulatory bacterial components such as LPS, but can also be induced by exposure to autologous immunomodulatory molecules such as HSP60 (Kilmartin and Reen 2004). The work ofKilmartin and Reen (2004) and Habich et al. (2005) is key to explaining a potential endotoxin tolerance state in MC children given the phenotypic evidence that HSP60 induces tolerance in monocytes similar to that seen with LPS, and that HSP60 “priming” down regulates CD86 and HLA-DR levels. Kilmartin and Reen suggested that the reorientation of monocyte function resulting from LPS exposure and/or autologous HSP60 is a mechanism by which the body is capable of controlling the instigation of a hyperinflammatory systemic response. Such “protection” has significant deleterious effects on the functional capacity of the immune system and could give rise to a state of “immunoparalysis,” reflecting a generalized in vivo deactivation of the entire cell-mediated innate immune system (Kilmartin and Reen 2004). Thus, the release of endogenous HSP, such as HSP60, into the extracellular environment may serve as a “danger signal” that initiates proinflammatory responses in the absence of foreign incursions and may subsequently play a critical role in monocyte deactivation and immunoparalysis in vivo (Chen et al. 1999; Kilmartin and Reen 2004).
Given the links between systemic inflammation and risk factors for cardiovascular pathology, these children might also be at risk of cardiovascular disease (Bräuner et al. 2008; Chuang et al. 2007; Mant et al. 2008; Rückerrl et al. 2007; Stoll et al. 2004). Atherosclerosis is recognized as a chronic inflammatory disease, and the lower LBP/sCD14 ratio, as we observed in this work, has been described in chronic subacute infections and higher risk of atherosclerosis (Stoll et al. 2004; Wiedermann et al. 1999).
MC children have evolved numerous mechanisms for attempting to control the constant state of inflammation related to their environmental exposures, and as part of these responses a picture of systemic inflammation, altered immune responses, and an endotoxin tolerance-like state is emerging with all the potential harmful results, including temporary immunodeficiency or even immunoparalysis (Mege et al. 2006). We recognize that serial assessment of NK cell cytotoxicity, cytokine responsiveness, NK cell subset phenotype, and cytokine production in MC children versus controls will provide a more complete understanding of NK cell status in these children (Orange 2008). More research is needed to elucidate causal pathways involving the effects of air pollution, immunodysregulation, and systemic inflammation in healthy children.
In summary, children exposed to significant concentrations of air pollutants including PM2.5 develop systemic inflammation and an alteration in their immune responses. C-reactive protein, a prototypic acute-phase reactant, and the prostaglandin E metabolite resulting from COX pathway activation have significant correlations with cumulative concentrations of PM2.5. These children have evolved several complex responses to the sustained exposures to air pollution, rendering them potentially more susceptible to infectious, malignant, and degenerative processes. MC children are potentially at risk of developing cancers, severe varicella infections, and atherosclerosis. Moreover, the systemic inflammation and the immunodysregulation observed in this study could play a role in the brain-altered immune responses and the cognitive deficits and structural brain changes observed in MC children (Calderón-Garcidueñas, Solt et al. 2008;Calderón-Garcidueñas, Mora-Tiscareño et al. 2008).
This study supports the concept that exposure to air pollution is a major threat to children’s health (Calderón-Garcidueñas, Villareal-Calderón et al. 2008; Calderón-Garcidueñas, Solt et al. 2008; Calderón-Garcidueñas, Mora-Tiscareño et al. 2008;Heinrich and Slama 2007; Kim 2004; Moshammer et al. 2006). Children represent a population that is uniquely vulnerable to adverse environmental factors, with disproportionally higher exposures relative to body weight compared to adults (Landrigan 2004; Landrigan et al. 2004). Furthermore, they represent the foundations of all future generations, making it essential to understand and protect them from adverse effects of environment on their current and future health.
Acknowledgments
We acknowledge the technical support of Raquel García and Silvia Monroy. This work was supported by: 1K01 NS046410-01A1, 1R21-ES013293-01A1, National Science Foundation 0346458 and the Montana Board of Research and Commercialization Technology Grant No. 04-06. This work was presented in part at the American Thoracic Society International Conference 2008, Toronto, Ontario, Canada by Rodolfo Villarreal-Calderón.
References