The liver is structurally and functionally complex and has been considered second only to brain in its complexity. Many mysteries still exist in this heterogeneous tissue whose functional unit of the lobule has continued to stump morphologists for over 300 years. The primary lobule, proposed by Matsumoto in 1979, has been gaining acceptance as the functional unit of the liver over other conceptual views because it’s based on vessel architecture and includes the classic lobule as a secondary feature. Although hepatocytes comprise almost 80% of the liver, there are at least another dozen cell types, many of which provide “cross-talk” and play important functional roles in the normal and diseased liver. The distribution and functional roles of all cells in the liver must be carefully considered in both the analysis and interpretation of research data, particularly data in the area of genomics and “phenotypic anchoring” of gene expression results. Discoveries regarding the functional heterogeneity of the various liver cell types, including hepatocytes, hepatic stellate cells, sinusoidal endothelia, and Kupffer cells, are providing new insights into our understanding of the development, prevention and treatment of liver disease. For example, functional differences along zonal patterns (centrilobular or periportal) have been demonstrated for sinusoidal endothelium, Kupffer cells, and hepatocytes and can explain the gradients and manifestations of disease observed within lobules. Intralobular gradients of bile uptake, glycogen depletion, glutamine synthetase, and carboxylesterase by hepatocytes; widened fenestrations in centrilobular sinusoidal lining cells; and differences in the components of centrilobular extracellular matrix or function of Kupffer cells have been demonstrated. Awareness of the complexities and heterogeneity of the liver will add to a greater understanding of liver function and disease processes that lead to toxicity, cancer, and other diseases.
INTRODUCTION
The liver is structurally and functionally heterogeneous and has been considered second only to brain in its complexity. Being the “main extracellular compartment” for adult vertebrates, the liver has thousands of vital functions including the efficient uptake of amino acids, carbohydrates, bile acids, cholesterol, proteins, lipids and vitamins for storage and metabolism subsequent to release into bile and/or blood (Bloom and Fawcett, 1975; Jones and Spring-Mills, 1983; LaBrecque, 1994; Burt and Day, 2002). The liver also has 2 distinct blood supplies, contains at least a dozen different cell types, regulates blood volume, and is the major site for biotransformation (particularly making hydrophobic molecules water soluble) and defense against foreign macromolecules and xenobiotics. Many aspects of liver function and structure still mystify biologists and pathologists and the 3-dimensional anatomy of the liver is poorly understood. Studies elucidating the 3-dimensional hepatic parenchymal and vascular structure are integral to our interpretation of genomics and proteomics data and furthering our understanding of the development and prevention of liver disease. Herein, we review the current understanding of the hepatic parenchymal architecture at the level of lobes, lobules, and individual cells as well as hepatic vasculature, functional units, and functional gradients. Last, applications of these concepts regarding phenotypic anchoring for genomics analysis and advances in imaging technology are discussed.
ANATOMY OF THE LIVER LOBES, LOBULES, AND CELLS
Lobes and Lobules
The liver is a heterogeneous tissue whose functional unit of the lobule has continued to stump morphologists for over 300 years (for review see MacSween et al., 2002). It is amazing that no matter the angle that a liver is sectioned, it basically has the same histologic appearance, i.e., multiple units with an hepatic venule (also known as central vein) centrally surrounded by about 4–6 portal areas. This phenomenon is the basis for the liver being referred to as having an “isotropic parenchyma” (Matsumoto and Kawakami, 1982) and it contributes to the mystifying, complex, 3-dimensional architecture. Attempts to understand the 3-dimensionality have helped us better understand liver function.
Mice and rats each have 4 liver lobes: median (or middle), left, right, and caudate and all, except the left, are further subdivided into 2 or more parts (Eustis et al., 1990; Harada et al., 1999; Kogure et al., 1999). Mice and humans have a gall bladder, but not the rat. Human liver lobes have traditionally be designated as right, left, quadrate, and caudate, but recently it has been proposed that the liver can be subdivided into 9 segments based on the vascular and ductal branching patterns to the right and left sides (Kogure et al., 1999; MacSween et al., 2002). This compartmental pattern is useful to understand lobar or intralobar degeneration related to disruption of the blood supply and to facilitate surgical resection. The hepatic lobes of the rat appear to have similar fundamental portal and hepatic venous systems, and thus segments, comparable to that of human liver (Kogure et al., 1999). The vascular systems to or from lobes show individual variations in humans as well as in rats (Kogure et al., 1999; MacSween et al., 2002). At any given moment the liver contains blood equivalent to approximately 25% of the cardiac output (Burt and Day, 2002). The portal vein and the hepatic artery are the two main vascular systems that supply blood to the liver. The portal vein supplies about 70% of the blood flow and 40% of the oxygen while the hepatic artery supplies 30% of the flow and 60% of the oxygen (Burt and Day, 2002). The portal blood drains from the mesenteric, gastric, splenic, and pancreatic veins and travels to the liver where it branches into the right and left sides of the liver. There can be incomplete mixing of blood coming from the gastrointestinal tract and spleen leading to variation in delivery of various nutrients, toxins, and other elements to the liver lobes (called portal streamlining) (Haywood, 1981; Faa et al., 1987, 1994, 1995; Thein et al., 2003; Dantel et al., 2004). For example, the blood draining the stomach and spleen tend to flow to the left side of the liver. Also localized or generalized core redistribution of blood flow or blood pooling is controlled by nerve stimulation (Stuart and Wheatley, 1995; Oakley et al., 2003) or hepatic stellate cells (Ratziu and Friedman, 1997; MacSween et al., 2002; Mabuchi et al., 2004) that can potentially lead to lobe variation in liver disease. Lobe variation has been reported for acetaminophen hepatotoxicity (Heinloth et al. 2004; Irwin et al. 2004a, 2004b), copper distribution (Haywood, 1981; Faa et al. 1987, 1995), iron and phosphorous (Ambu et al., 1995), chemical carcinogenesis (Solt et al., 1977; Richardson et al., 1986), cirrhosis (Matsuzaki et al., 1997; Regev et al., 2002), and regeneration (LaBrecque, 1994). The conducting portal vessels deliver blood to the parenchymal vessels called preterminal and terminal portal venules, respectively. Blood from the terminal portal venules enters the sinusoids (Matsumoto and Kawakami, 1982). The hepatic artery generally accompanies the portal veins in the portal triads and its smaller branches feed the sinusoids at varying levels and biliary tracts (which most often subsequently drains into sinusoids; a so-called portal-portal flow) (MacSween et al., 2002). The sinusoidal blood flow is carefully regulated (Ekataksin and Kaneda, 1999; McCuskey, 2000) and collects into terminal hepatic venules (also called central veins) prior to emptying into larger hepatic veins and eventually to the vena cava. Lymph fluid accumulates in the space of Disse and periportal tissue of Mall before draining into lymphatic vessels in the portal canal and on to hilar lymphatic channels and eventually to the thoracic duct. The portal triad is defined by the portal vein, bile duct, and hepatic artery, however the portal area contains on average about 6 profiles (range is 2–35) with an average of 1–2 arteries, 1 portal vein, 1–2 bile ducts, lymphatics, nerves in a connective tissue matrix comprised mainly by type 1 collagen (MacSween et al., 2002).
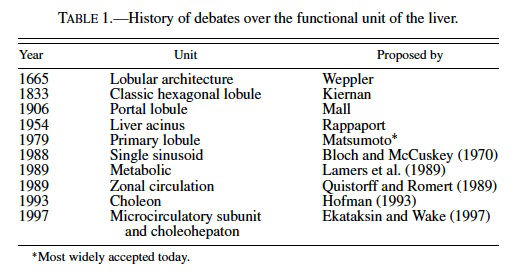
The lobular pattern of the liver was first noted by Weppler in 1665 and over the last century the functional unit of the liver has been hotly debated (MacSween et al., 2002). Kiernan described the classic hexagonal lobule in 1833 (Kiernan, 1833), and about 70 years later Mall proposed the portal lobule (Mall, 1906) (Table 1). The portal lobule is based on the portal vessels supplying the lobule centrally and being drained at the periphery. This concept was based on studies in the dog and rabbit. In 1954, Rappaport proposed the liver acinus as the functional unit (Rappaport et al., 1954), and it was based on the zones or hepatocytes between central veins. The liver acinus was distinct because it represented both a functional and structural unit that allowed for the explanation of lesions such as bridging necrosis and fibrosis. The primary lobule, proposed by Matsumoto et al. in 1979, has been gaining acceptance as the functional unit of the liver over other conceptual views because it’s based on vessel architecture and includes the classic lobule as a secondary feature (Matsumoto et al., 1979; Matsumoto and Kawakami, 1982). It is based on the conducting and parenchymal portion of portal venous tree. The 3-dimensional shape of the individual lobules are not cylindrical or prismatic but tortuous and branching, and the liver tends to grow by division of the classic lobule. Although a number of alternative functional hepatic units were proposed during the 1980s and 1990s, such as the single sinusoidal, metabolic, zonal circulation, choleon, microcirculatory, and choleohepaton units (see MacSween, 2002 for review), the Matsumoto model remains the most widely accepted. Matsumoto and colleagues demonstrated intricate details of branching and ramification of the portal vasculature in the context of the lobule by studying thousands of serial sections of 2 human cadaver livers. Teutsch et al. (1999) used a similar approach in reconstructing the 3-dimensional structure of the rat liver. Using immunohistochemistry of glucose- 6-phosphatase (which has a gradient of expression being at greater levels in the periportal hepatocytes compared to centrilobular cells) to define lobules, they examined 146 serial sections of rat liver to define a secondary unit containing 14 primary lobules. The nomenclature is different in the rat study in that the Matsumoto primary lobule of the human equates to the rat secondary lobule describe by Teutsch. The rat secondary unit is supplied by portal vessels at the surface and drained centrally by a common single hepatic venular tree, similar to the Matsumoto model of humans (Matsumoto and Kawakami, 1982; Teutsch et al., 1999). Teutsch et al. also found no evidence of septal branches of portal veins in the rat liver typical of those seen in humans or pigs, suggesting that the concept of the liver acinus or portal unit cannot be applied in the rat. Bhunchet and Wake (1998) examined the rat and pig lobular architecture and concluded that the rat does not appear to have supplying portal vessels as described in pig and humans but rather a polyhedral portal unit with fewer terminal portal branches and sinusoids that terminate in all branches of the central vein. Additionally they found that the rat, as opposed to humans, does not have portal veins on the surface of the liver. They propose that the rat liver lobular unit is more similar to that of the dog and rabbit and all 3 species differ from the human and the pig (Bhunchet and Wake, 1998). It is doubtful that the classic lobule and the acinus coexist in the rat liver.
Cells of the Liver
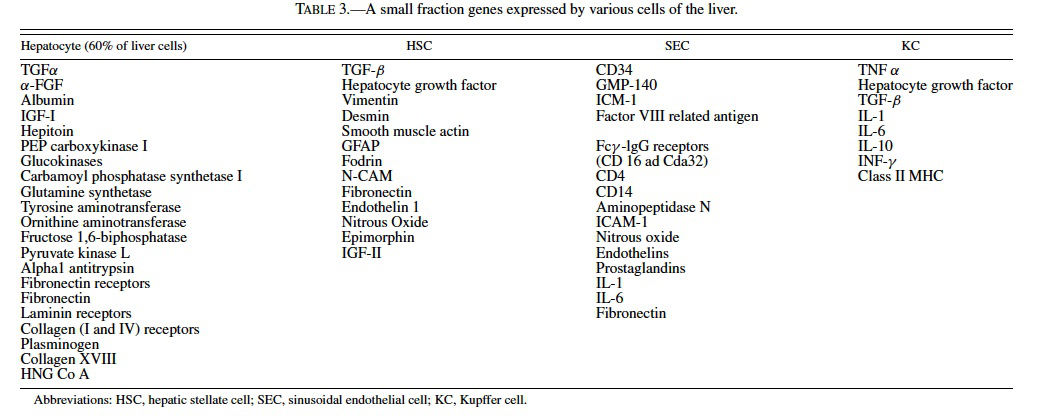
At least 15 different cell types can be found in normal liver (Table 2) (Bloom and Fawcett, 1975; Jones and Spring-Mills, 1983; Kmiec, 2001; Burt and Day, 2002; Malik et al., 2002). Hepatocytes are the most numerous and comprise 60% of the total cells and 80% of the volume of liver. Sinusoidal endothelial cells (SECs), Kupffer cells, hepatic stellate cells (HSC), and biliary epithelium make up a significant number (3–20% each) of the remaining biologically important cells.
Hepatocytes are arranged in plates or laminae of cords 1 cell thick (called muralium) that branch and anastomose in a continuous labrynth with limiting plates being at the capsule and portal regions (MacSween et al. 2002). The 6 or more surfaces of the hepatocyte either abut adjacent parenchymal cells, border bile canaliculi, or are exposed to the perisinusoidal space (this surface being covered by microvilli). Being the workhorses of the liver, hepatocytes contain the machinery necessary to carry out the thousands of vital functions. Normally, about 15% of the cell volume is composed of smooth and rough endoplasmic reticulum and there are about 30 lysosomes and 500 peroxisomes (microbodies) per cell (Bloom and Fawcett, 1975; Jones and SpringMills, 1983; MacSween et al., 2002). The mitochondria number about 1,000 per hepatocyte and there are numerous free ribosomes, golgi complex, cytoskeleton elements (such as microfilaments, intermediate filaments, and microtubules), and varying levels of cytoplasmic lipid and glycogen. One of the main hepatocyte functions is the production of bile, which averages about 15 ml/kg/day in humans (Jones and SpringMills, 1983). With age the number of hepatocytes decreases and hypertrophy, polyploidy, lysosomes, and smooth endoplasmic reticulum increases (Jones and Spring-Mills, 1983). The mitochondria and microbodies remain unchanged with age and the microsomal drug-metabolizing capabilities decrease.
Biliary epithelium primarily acts as a lining of the conduit for bile flow but it also modifies canalicular bile and concentrates bile in the gall bladder. Biliary epithelia are “effective communicators” with neighboring cells in producing mediators that are involved in cell growth and response to injury.
The sinusoidal endothelial cells (SECs) are the primary barrier between blood and hepatocytes and they act to filter fluids, solutes, and particles between the blood and space of Disse (Braet and Wisse, 2002; Smedsrod, 2004) and represent up to 20% of the liver cells. SECs are a unique type of endothelial cells in that they have fenestrae (first discovered in 1970), lack a basal lamina, and can transfer molecules and particles by endocytosis (Braet and Wisse, 2002). Blood cells pass through sinusoids that gently “massage” fluids through fenestrae and, being dynamically active, it is not surprising that SECs contain extensive cytoskeletal support. Fenestrae alterations can play a critical role in disease including atherosclerosis, cirrhosis, and implantation of tumor metastases (Braet and Wisse, 2002; Smedsrod, 2004). If fenestrae are relatively small, then less cholesterol is removed from circulation and accumulates in blood increasing the risk for atherosclerosis development. For example, rabbits have relatively smaller fenestrae compared to some other species such as the rat and are at increased risk for the development of atherosclerosis. It has been shown that drugs that dilate fenestrae decrease the occurrence of atherosclerosis. Defenestration is thought to play a role in some hepatoxin-induced cirrhosis.
Kupffer cells represent 15% of the liver cells (30% of sinusoidal cells) and are derived from circulating monocytes (Jones and Spring-Mills, 1983; MacSween et al., 2002; Bykov et al., 2004). They can proliferate locally, are phagocytic, are the major producers of cytokines as mediators of inflammation and provide “cross-talk” with other cells. Hepatic stellate cells (HSC) comprise about 5% of liver cells and were previously called Ito or fat-storing cells. They were first discovered by Wilhelm Kupffer in the late 1800s (Wake, 2004) and are known to be a major player in regeneration and hepatic fibrogenesis and cirrhosis (Ratziu and Friedman, 1997; Fehrenbach et al., 2001; Albanis et al., 2003; Kawada, 2004; Mabuchi et al., 2004). HSC normally produce extracellular matrix, control microvascular tone, store and metabolize vitamin A and lipid, and when activated transform to myofi- broblasts. In the activated myofibroblast form, they typically express desmin and smooth muscle actin filaments.
The extracellular matrix (ECM) is important in the regulation and modulation of hepatic function. Five to 10% of the liver is collagen. The ECM has numerous components including matrix metalloproteinases; the glycoproteins laminin, fibronectin, vitronectin, undulin, nidogen (entactin); and proteoglycans such heparan sulfate (Burt and Day, 2002; MacSween et al., 2002).
Functional Gradients
Consideration of gradient differences in cell and matrix composition (i.e., enzyme activities) of the liver (Germain et al., 1987; Gebhardt, 1992; Teutsch et al., 1999; Burt and Day, 2002; MacSween et al., 2002) are important when evaluating gene and protein changes as measured by genomic and proteomic methods. Furthermore, these different functional properties between periportal and centrilobular cells and matrix can also help explain the regional distribution of lesions and susceptibility of cells to certain hepatotoxicants. Not only do hepatocytes have gradients of gene and protein activity that varies from the periportal (PP) region to the centrilobular (CL) region, but gradients also exist for SECs, Kupffer cells, HSC, and the matrix in the space of Disse (Gebhardt, 1992; Lindros et al., 1997; Braet and Wisse, 2002; MacSween et al., 2002; Bykov et al., 2004).
Functional gradients of hepatocytes have been intensely studied and reported. PP hepatocytes normally have higher levels of oxygen saturation (twice that of CL hepatocytes), peroxisomes, glucose-6-phosphatase (G6P) activity, urea cycle activity, bile acid uptake, glutathione content, and glycogen synthesis, which precedes that in the CL area (Gebhardt, 1992; MacSween et al., 2002). For glycogen, the periportal region is the first area depleted and restored after starvation conditions. There is an intralobular gradient of bile acid uptake with midzonal and CL hepatoctyes having increased uptake during periods of greater load. Glutamine synthetase is exclusively expressed in CL hepatocytes while enzymes like glucokinase (GK), carboxylesterase, and ethanol-induceable cyp 2E1 are at higher levels in CL hepatocytes compared to PP hepatocytes. Teutsch et al. (1999) demonstrated the importance of determining volumetric gradients for quantifying G6P and GK gradients in rat liver. For an enzyme such as G6P, that has greater activity in periportal zones, the ratio of activity is about 1.7 to 1 comparing the level in PP to CL hepatocytes in a linear fashion. If one considers the 2-dimensional volume on a section of a classic lobule, then there are many more PP compared to CL hepatocytes and the ratio based on volume is much greater at 8.1 to 1. In contrast, GK is higher in the CL region and the liner ratio is 3.4 to 1 compared to a volumetric ratio of 0.7 to 1.
Many components of the liver, other than hepatocytes, have gradients. For example, the fenestrae in SECs are greater in number and diameter near the central veins (Braet and Wisse, 2002). Periportal Kupffer cells are larger and more phagocytically active in periportal regions (Bykov et al., 2004) which makes biological sense as an early line of defense. HSCs properties vary along 3 areas, the periportal, midzone, and central vein (Ratziu and Friedman, 1997). In the periportal region, HSCs are smaller, have perisinusoidal processes, and a small volume of lipid droplets while in the midzone region they are elongate with a large volume of lipid and greater abundance of desmin and in the centrilobular region and intercellular processes, relatively more vitamin A, and reduced amounts of desmin. In the periportal region the space of Disse has greater amounts of laminin, type IV collagen, and heparin sulfate. In the centrilobular region it has fibronectin, type III collagen, and dermatan sulfate (MacSween et al., 2002).
LIVER TRANSCRIPTOME AND PHENOTYPIC ANCHORING
The liver transcriptome (i.e., genes expressed as measured by mRNA) is believed only second to the brain in its complexity and includes about 25–40% (Shackel et al. 2002) of the approximately 50,000 mammalian genes (Table 3). During disease states the transcriptome can double or triple and its increased complexity is due not only to differential gene expression (up- and down-regulation of genes) but also to the mRNA contributions from the heterogeneous cell populations in the liver. When one considers that over a dozen cell types comprise the liver in varying proportions, particularly in disease states, knowledge about the cell types and cellspecific gene expression profiles help unravel the complex genomic and proteomic data sets. For example, hepatocytes, which comprise over 60% of the liver cells (MacSween et al., 2002), probably contribute the most diverse and numerous transcriptome normally and in disease states. In the case of cirrhosis, the cell makeup of the liver changes and the hepatic stellate cell and other fibrogenic cells are likely making greater contributions to the mRNA pool being studied. This led to a need to better characterize the histological cellular components of the tissues from which mRNA and protein is extracted, a concept now referred to as “phenotypic anchoring” (Tennant, 2002).
“Phenotypic anchoring,” a recently coined term often used in reference to toxicogenomics studies (Tennant, 2002; Paules, 2003; Waters et al., 2003), refers to the linkage of “patterns of gene expression to specific parameters of well-defined, conventional indices of toxicity.” The establishment of such linkages, particularly to histologic alterations, will help define the incidental from causal effects of a compound and delineate disease pathogenesis and cell types that contribute to the gene or protein expression profiles. An early study that took such an approach is that of Hamadeh et al. (2002). They studied the gene expression profiles of acute liver toxicity for the nongenotoxic rat hepatocarcinogen, methapyrilene, an antihistamine now banned in the United States and other countries. Methapyrilene was administered to 7-week-old rats at 10 or 100 mg/kg/day for 1, 3, or 7 days. Histologically, there was individual hepatocyte necrosis accompanied by mild-to-moderate lymphocytic inflammation in the periportal regions that increased with dose and time. Their transcriptome results reflected not only dose and time effects but also provided evidence that suggested the gene expression profiles were dependent on the histological composition of the samples.
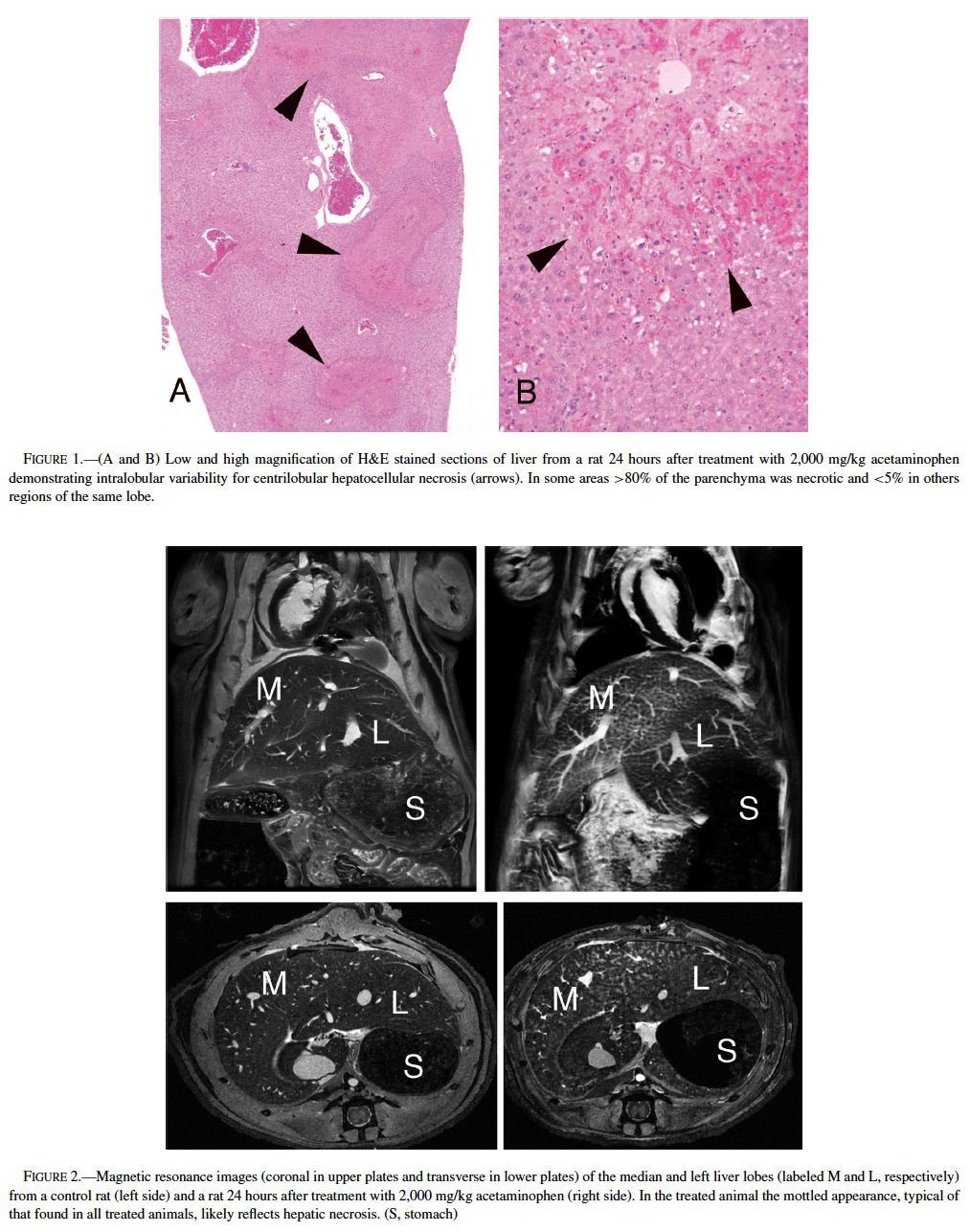
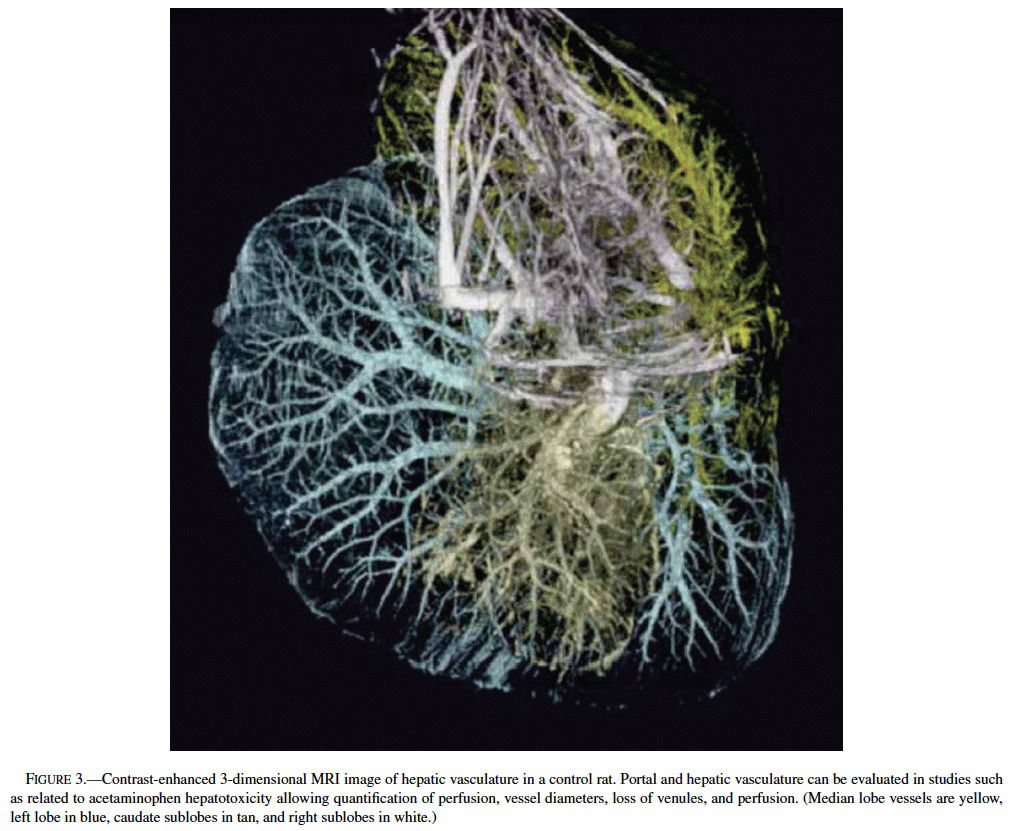
Acetaminophen, a model hepatotoxicant under study to assess the strengths and weaknesses of genomics and proteomics technologies (Heinloth et al., 2004; Irwin et al., 2004a, 2004b), also is a good example for understanding and utilizing “phenotypic anchoring” to better understand genomics data. Previous studies have revealed that there is an unexplained and striking inter- and intralobular variability of acute hepatic necrosis with some regions having massive necrosis and adjacent areas within the lobe or other lobes from the same animal showing no injury at all (Figure 1) (Irwin et al., 2004b). Variability between lobes has previously been observed in the severity of acetaminophen toxicity (Heinloth et al., 2004; Irwin et al., 2004a, 2004b), copper distribution (Haywood, 1981; Faa et al., 1987, 1995), iron and phosphorous (Ambu et al., 1995), chemical and spontaneous carcinogenesis (Solt et al., 1977; Richardson et al., 1986; Elba et al., 2002; Garcia-Torres et al., 2003), cirrhosis (Matsuzaki et al., 1997; Regev et al., 2002), and regeneration (LaBrecque, 1994). The mechanism is often uncertain, however factors such as portal streamlining of blood to the liver (Duchen, 1961; Daniel et al., 2004), redistribution of blood to core of the liver secondary to nerve stimulation (Stuart and Wheatley, 1995), and exposures during fetal development (when the umbilical vein is patent) (Zhang and Byrne, 2000), and possibly lobar gradients (Germain et al., 1987) are important. Studies are currently underway at the National Toxicology Program (NTP) to better define the lobe variability for acetaminophen toxicity in the context of gene expression profiles. The distribution of toxic insult may not be correctly assessed with random sampling of liver tissue for microarray gene expression analysis. We are utilizing magnetic resonance imaging (MRI) technology to determine whether hepatic centrilobular necrosis and/or vascular damage induced by acetaminophen is apparent in MRI images of the rat liver with the hopes of identifying lobe variation in necrosis and directly correlating light microscopic alterations with MRI findings. Magnetic resonance microscopy (MRM) is fast becoming a valuable tool to visualize histologic alterations in whole animals (Delnomdedieu et al., 1996; Johnson et al., 2002). Six male Fisher 344 rats approximately 7–8 weeks of age (150–210 gm) were treated with a single dose of 2,000 mg/kg of acetaminophen by gavage, 3 control rats received 0.5% ethyl cellulose vehicle only by gavage. Under deep anesthesia (Nembutal and butorphanol, IP), animals were perfused via a transcardial approach first with heparinized saline to exsanguinate and then with a mixture of neutral buffered formalin and paramagnetic contrast agent (Magnevist). MRM imaging was performed at the Center for In Vivo Microscopy, a National Institutes of Health National Center for Research Resources (NIH/NCRR) and involved the use of a 7.0 Tesla magnet using a 3D spin warp encoding with TR = 100 ms, TE = 5 ms. The MRM liver images for each rat were reviewed without knowledge of treatment. By MRM, all 6 treated rats had an irregular pattern of mottled parenchymal alterations throughout most liver lobes that were not evident in the 3 control rats (Figure 2). Three-dimensional contrast-enhanced images of the hepatic venous (portal and hepatic central) vascular system (Figure 3) has allowed for evaluation of any potential inter- and intralobular vascular damage (i.e., to the hepatic/central veins and venules). Further studies are under way to make direct correlations of the MRM lesions with the histologic findings for each liver lobe (median, left, right, and caudate) and to further assess the existence of intralobe variation.
CONCLUSIONS
The liver is a heterogeneously complex organ and understanding the 3-dimensional structure, physiology, and cellular components is integral to the interpretation of genomic and proteomic data as well as disease pathogenesis and prevention. Future elucidation of the heterogeneity and complexity of the liver, for example using emerging genomics and imaging technology, will help in the fight against liver disease and cancer and find new targets for therapy. New technologies and approaches such as gene expression microarray analysis, proteomics, phenotypic anchoring, and MRI imaging will greatly aid in acquiring such knowledge.
ACKNOWLEDGMENTS
We appreciate the assistance from Mr. Bryn Forbes from MRPath, Durham, North Carolina; Dr. G. Allan Johnson of Duke University Medical Center; and Ms. Jennifer Spedick of North Carolina State University College of Veterinary Medicine for their assistance in generating and evaluating the rat liver MRI images. We also appreciate the careful review and feedback on the manuscript provided by Drs. Georgette Hill and Rick Irwin from NIEHS.
REFERENCES
Albanis, E., Safadi, R., and Friedman, S. L. (2003). Treatment of hepatic fibrosis: almost there. Curr Gastroenterol Rep 5, 48–56.
Ambu, R., Crisponi, G., Sciot, R., Van Eyken, P., Parodo, G., Iannelli, S., Marongiu, F., Silvagni, R., Nurchi, V., Costa, V., and et al. (1995). Uneven hepatic iron and phosphorus distribution in beta-thalassemia. J Hepatol 23, 544–9.
Bhunchet, E., and Wake, K. (1998). The portal lobule in rat liver fibrosis: a re-evaluation of the liver unit. Hepatology 27, 481–7.
Bloch, E. H. (1970). The termination of hepatic arterioles and the functional unit of the liver as determined by microscopy of the living organ. Ann NY Acad Sci 170, 78–87.
Bloom, W., and Fawcett, D. W. (1975). Liver and gall bladder. In A textbook of histology (W. Bloom and D. W. Fawcett, eds.), pp. 688–718. W. B. Saunders, Philadelphia.
Braet, F., and Wisse, E. (2002). Structural and functional aspects of liver sinusoidal endothelial cell fenestrae: a review. Comp Hepatol 1, 1.
Burt, A. D., and Day, C. P. (2002). Pathophysiology of the liver. In Pathology of the Liver (R. N. M. MacSween, A. D. Burt, B. C. Portmann, K. G. Ishak, P. J. Scheuer and P. P. Anthony, eds.), pp. 67–105. Churchill Livingstone, New York.
Bykov, I., Ylipaasto, P., Eerola, L., and Lindros, K. O. (2004). Functional differences between periportal and perivenous Kupffer cells isolated by digitonin-collagenase perfusion. Comp Hepatol 3 (Suppl 1), S34.
Daniel, G. B., DeNovo, R. C., Sharp, D. S., Tobias, K., and Berry, C. (2004). Portal streamlining as a cause of nonuniform hepatic distribution of sodium pertechnetate during per-rectal portal scintigraphy in the dog. Vet Radiol Ultrasound 45, 78–84.
Delnomdedieu, M., Hedlund, L. W., Johnson, G. A., and Maronpot, R. R. (1996). Magnetic resonance microscopy—a new tool for the toxicologic pathologist. Toxicol Pathol 24, 36–44.
Duchen, L. W. (1961). The effects of deprivation of portal blood on the liver and its influence on carbon tetrachloride liver injury in the rat. Br J Exp Pathol 42, 247–52.
Ekataksin, W., and Kaneda, K. (1999). Liver microvascular architecture: an insight into the pathophysiology of portal hypertension. Semin Liver Dis 19, 359–82.
Ekataksin, W., and Wake, K. (1997). The anatomy and physiology of the liver. In Progress in Liver Diseases (J. L. Boyer and R. K. Ockner, eds.), pp. 1–30. W. B. Saunders, Philadelphia.
Elba, S., Buongiorno, G. P., Caruso, M. L., Noviello, M. R., and Manghisi, O. G. (2002). Main characteristics of hepatocellular carcinoma and cirrhosis and therapeutic approaches. Curr Pharm Des 8, 1007–11.
Eustis, S. L., Boorman, G. A., Harada, T., and Popp, J. A. (1990). Liver. In Pathology of the Fischer Rat (G. A. Boorman, S. L. Eustis, M. R. Elwell, C. A. Montgomery and W. F. Mackenzie, eds.), pp. 71–74. Academic Press, San Diego.
Faa, G., Liguori, C., Columbano, A., and Diaz, G. (1987). Uneven copper distribution in the human newborn liver. Hepatology 7, 838–42.
Faa, G., Nurchi, V., Demelia, L., Ambu, R., Parodo, G., Congiu, T., Sciot, R., Van Eyken, P., Silvagni, R., and Crisponi, G. (1995). Uneven hepatic copper distribution in Wilson’s disease. J Hepatol 22, 303–8.
Faa, G., Sciot, R., Farci, A. M., Callea, F., Ambu, R., Congiu, T., van Eyken, P., Cappai, G., Marras, A., Costa, V., and et al. (1994). Iron concentration and distribution in the newborn liver. Liver 14, 193–9.
Fehrenbach, H., Weiskirchen, R., Kasper, M., and Gressner, A. M. (2001). Upregulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofi- broblasts. Hepatology 34, 943–52.
Garcia-Torres, M. L., Zaragoza, A., Giner, R., Primo, J., and del Olmo, J. A. (2003). Incidence and epidemiological factors of hepatocellular carcinoma in Valencia during the year 2000. Rev Esp Enferm Dig 95, 381–8.
Gebhardt, R. (1992). Metabolic zonation of the liver: regulation and implications for liver function. Pharmacol Ther 53, 275–354.
Germain, A., Garrido, A., Canas, P., Llanos, A., and Valenzuela, A. (1987). Differences in the lipid peroxidative status, cytochrome P-450 content and microsomal oxygen consumption between right and left lobes of the liver in fetal sheep. Comparison with maternal liver. Biochem Int 15, 571– 7.
Hamadeh, H. K., Knight, B. L., Haugen, A. C., Sieber, S., Amin, R. P., Bushel, P. R., Stoll, R., Blanchard, K., Jayadev, S., Tennant, R. W., Cunningham, M. L., Afshari, C. A., and Paules, R. S. (2002). Methapyrilene toxicity: anchorage of pathologic observations to gene expression alterations. Toxicol Pathol 30, 470–82.
Harada, T., Enomoto, A., Boorman, G. A., and Maronpot, R. R. (1999). Liver and gallbladder. In Pathology of the mouse (R. R. Maronpot, ed.), pp. 119–126. Cache River Press, Vienna, IL.
Haywood, S. (1981). The non-random distribution of copper within the liver of rats. Br J Nutr 45, 295–300.
Heinloth, A. N., Irwin, R. D., Boorman, G. A., Nettesheim, P., Fannin, R. D., Sieber, S. O., Snell, M. L., Tucker, C. J., Li, L., Travlos, G. S., Vansant, G., Blackshear, P. E., Tennant, R. W., Cunningham, M. L., and Paules, R. S. (2004). Gene expression profiling of rat livers reveals indicators of potential adverse effects. Toxicol Sci 80, 193–202.
Hofmann, A. F. (1993). The choleohepatic circulation of unconjugated bile acids: an update. In Bile acids and the hepatobiliary system: from basic science to clinical practice, (P. G., S. A. and W. Gerok, eds.), pp. 143–160. Kluwer Academic Publishers, Dordrecht.
Irwin, R. D., Boorman, G. A., Cunningham, M. L., Heinloth, A. N., Malarkey, D. E., and Paules, R. S. (2004a). Application of toxicogenomics to toxicology: basic concepts in the analysis of microarray data. Toxicol Pathol 32 (Suppl 1), 72–83.
Irwin, R. D., Parker, J. P., Lobenhofer, E. K., Burka, L. T., Blackshear, P. E., Vallant, M. K., Lebetkin, E. H., Gerken, D. F., and Boorman, G. A. (2004b). Transcriptional profiling of the left and median liver lobes of male F344 rats following exposure to acetaminophen. Toxicological Pathology. In press.
Johnson, G. A., Cofer, G. P., Fubara, B., Gewalt, S. L., Hedlund, L. W., and Maronpot, R. R. (2002). Magnetic resonance histology for morphologic phenotyping. J Magn Reson Imaging 16, 423–9.
Jones, A. L., and Spring-Mills, E. (1983). The liver and gallbladder. In Histology (L. Weiss, ed.), pp. 707–735. Elsevier Biomedical, New York.
Kawada, N. (2004). Molecular mechanism of stellate cell activation and therapeutic strategy for liver fibrosis. Comp Hepatol 3 (Suppl 1), S3.
Kiernan, F. (1833). The anatomy and physiology of the liver. Phil Trans R Soc Lond 123, 711–770.
Kmiec, Z. (2001). Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol 161, III–XIII, 1–151.
Kogure, K., Ishizaki, M., Nemoto, M., Kuwano, H., and Makuuchi, M. (1999). A comparative study of the anatomy of rat and human livers. J Hepatobiliary Pancreat Surg 6, 171–5.
LaBrecque, D. (1994). Liver regeneration: a picture emerges from the puzzle. Am J Gastroenterol 89, S86–96.
Lamers, W. H., Hilberts, A., Furt, E., Smith, J., Jonges, G. N., van Noorden, C. J., Janzen, J. W., Charles, R., and Moorman, A. F. (1989). Hepatic enzymic zonation: a reevaluation of the concept of the liver acinus. Hepatology 10, 72–6.
Lindros, K. O., Oinonen, T., Issakainen, J., Nagy, P., and Thorgeirsson, S. S. (1997). Zonal distribution of transcripts of four hepatic transcription factors in the mature rat liver. Cell Biol Toxicol 13, 257–62.
Mabuchi, A., Mullaney, I., Sheard, P., Hessian, P., Zimmermann, A., Senoo, H., and Wheatley, A. M. (2004). Role of hepatic stellate cells in the early phase of liver regeneration in rat: formation of tight adhesion to parenchymal cells. Comp Hepatol 3 (Suppl 1), S29.
MacSween, R. N. M., Desmet, V. J., Roskams, T., and Scothorne, R. J. (2002). Developmental anatomy and normal structure. In Pathology of the Liver (R. N. M. MacSween, A. D. Burt, B. C. Portmann, K. G. Ishak, P. J. Scheuer and P. P. Anthony, eds.), pp. 1–66. Churchill Livingstone, New York.
Malik, R., Selden, C., and Hodgson, H. (2002). The role of non-parenchymal cells in liver growth. Semin Cell Dev Biol 13, 425–31.
Mall, F. B. (1906). A study of the structural unit of the liver. Am J Anat 5, 227–308.
Matsumoto, T., and Kawakami, M. (1982). The unit-concept of hepatic parenchyma—a re-examination based on angioarchitectural studies. Acta Pathol Jpn 32 (Suppl 2), 285–314.
Matsumoto, T., Komori, R., Hamadeh, H. K., and et al. (1979). A study on the normal structure of human liver, with special reference to its angioarchitecture. Jikeikai Med J 26, 1–40.
Matsuzaki, S., Onda, M., Tajiri, T., and Kim, D. Y. (1997). Hepatic lobar differences in progression of chronic liver disease: correlation of asialoglycoprotein scintigraphy and hepatic functional reserve. Hepatology 25, 828–32.
McCuskey, R. S. (2000). Morphological mechanisms for regulating blood flow through hepatic sinusoids. Liver 20, 3–7.
Oakley, F., Trim, N., Constandinou, C. M., Ye, W., Gray, A. M., Frantz, G., Hillan, K., Kendall, T., Benyon, R. C., Mann, D. A., and Iredale, J. P. (2003). Hepatocytes express nerve growth factor during liver injury: evidence for paracrine regulation of hepatic stellate cell apoptosis. Am J Pathol 163, 1849–58.
Paules, R. (2003). Phenotypic anchoring: linking cause and effect. Environ Health Perspect 111, A338–9.
Quistorff, B., and Romert, P. (1989). High zone-selectivity of cell permeabilization following digitonin-pulse perfusion of rat liver. A re-interpretation of the microcirculatory zones. Histochemistry 92, 487–98.
Rappaport, A. M., Borowy, Z. J., Lougheed, W. M., and Lotto, W. N. (1954). Subdivision of hexagonal liver lobules into a structural and functional unit. Anat Rec 119, 11–34.
Ratziu, V., and Friedman, S. L. (1997). Pathobiology of hepatic stellate cells. In Functional Heterogeneity of Liver Tissue: From Cell Lineage Diversity to Sublobular Compartment-Specific Pathogenesis (F. Vidal-Vanaclocha, ed.), pp. 133–160. R. G. Landes Company, Austin.
Regev, A., Berho, M., Jeffers, L. J., Milikowski, C., Molina, E. G., Pyrsopoulos, N. T., Feng, Z. Z., Reddy, K. R., and Schiff, E. R. (2002). Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol 97, 2614–8.
Richardson, F. C., Boucheron, J. A., Dyroff, M. C., Popp, J. A., and Swenberg, J. A. (1986). Biochemical and morphologic studies of heterogeneous lobe responses in hepatocarcinogenesis. Carcinogenesis 7, 247–51.
Shackel, N. A., Gorrell, M. D., and McCaughan, G. W. (2002). Gene array analysis and the liver. Hepatology 36, 1313–25.
Smedsrod, B. (2004). Clearance function of scavenger endothelial cells. Comp Hepatol 3 (Suppl 1), S22.
Solt, D. B., Hay, J. B., and Farber, E. (1977). Comparison of the blood supply to diethylnitrosamine-induced hyperplastic nodules and hepatomas and to the surrounding liver. Cancer Res 37, 1686–91.
Stuart, E. T., and Wheatley, A. M. (1995). Redistribution of portal venous but not hepatic arterial flow is induced by hepatic nerve stimulation in the perfused rat liver. Arch Physiol Biochem 103, 99–108.
Tennant, R. W. (2002). The National Center for Toxicogenomics: using new technologies to inform mechanistic toxicology. Environ Health Perspect 110, A8–10.
Teutsch, H. F., Schuerfeld, D., and Groezinger, E. (1999). Three-dimensional reconstruction of parenchymal units in the liver of the rat. Hepatology 29, 494–505.
Thein, E., Becker, M., Anetzberger, H., Hammer, C., and Messmer, K. (2003). Direct assessment and distribution of regional portal blood flow in the pig by means of fluorescent microspheres. J Appl Physiol 95, 1808–16.
Wake, K. (2004). Karl Wilhelm Kupffer and his contributions to modern hepatology. Comp Hepatol 3 (Suppl 1), S2.
Waters, M. D., Olden, K., and Tennant, R. W. (2003). Toxicogenomic approach for assessing toxicant-related disease. Mutat Res 544, 415–24.
Zhang, J., and Byrne, C. D. (2000). Differential hepatic lobar gene expression in offspring exposed to altered maternal dietary protein intake. Am J Physiol Gastrointest Liver Physiol 278, G128–36.