The lung is the second most common target site of neoplasia of chemicals tested by the National Toxicology Program (NTP). Of all peer-reviewed NTP studies to date (N = 545), a total of sixty-four chemicals in sixty-six reports produced significant site-specific neoplasia in the lungs of rats and/or mice. Of the studies associated with lung tumor induction, approximately 35% were inhalation and 35% were gavage studies, with dosed-feed, dosed-water, topical, intraperitoneal, or in utero routes of chemical administration accounting for 18%, 6%, 3%, 1%, and 1% of the studies, respectively. The most commonly induced lung tumors were alveolar/bronchiolar (A/B) adenoma and/or carcinoma for both species. The most frequently observed nonneoplastic lesions included hyperplasia and inflammation in both species. The liver was the most common primary site of origin of metastatic lesions to the lungs of mice; however, skin was most often the primary site of origin of metastatic lesions to the lungs of rats. In summary, A/B adenoma and carcinoma were the most frequently diagnosed chemically induced tumors in the lungs of both rats and mice in the NTP toxicology and carcinogenesis bioassays, and hyperplasia and inflammation were the most common nonneoplastic changes observed.
INTRODUCTION
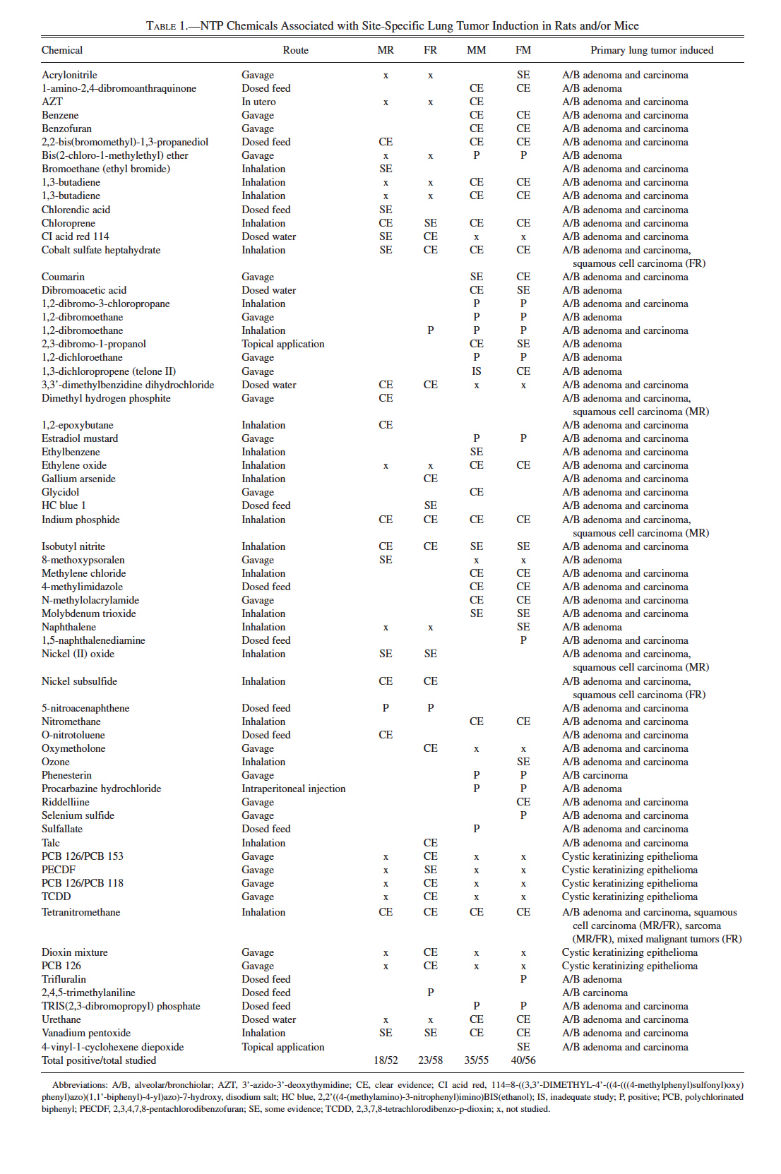
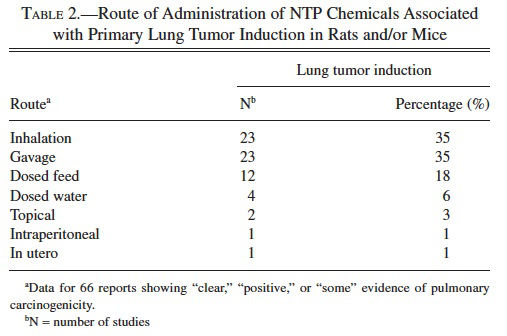
In the National Toxicology Program (NTP) two-year rodent bioassay, the lung is second to the liver as the most common target site of neoplasia of chemical carcinogens in male and female mice. In rats, the lung is third in females after the liver and mammary glands, and it is uncommonly involved in male rats. Of the 545 peer-reviewed NTP reports to date, sixty-six have identified chemical-related induction of primary pulmonary neoplasia. The most common routes of chemical exposure associated with site-specific lung tumor induction in NTP bioassays are inhalation and gavage (both 35%) followed by dosed-feed (18%); dosed-water, topical, intraperitoneal, and in utero exposures combined account for 11%.
The most common lung tumors induced in rats and mice by chemicals tested in the NTP bioassay are alveolar/bronchiolar (A/B) adenomas and carcinomas. The cell(s) of origin of most rodent lung tumors remains controversial. Many investigators have described tumor cells with features of alveolar type II cells, Clara cells, or both cell types in spontaneous and chemically induced pulmonary neoplasms in mice and rats (Belinsky et al. 1991; Dixon and Maronpot 1991; Herbert et al. 1994; Kauffman 1981; Kauffman et al. 1979; Nikitin et al. 2004; Ohshima et al. 1985; Parsa and Kauffman 1983; Rehm et al. 1989; Sato and Kauffman 1980; Ward et al. 1985). Since the cell(s) of origin for most rodent pulmonary neoplasms is debatable, the NTP classifies tumors originating in the alveolobronchiolar regions of the lung of rats and mice as A/B tumors. The occurrence of other tumor types in the lungs of rats and mice are less frequent; the diagnosis of squamous cell carcinoma and cystic keratinizing epithelioma are the next two most commonly induced tumor types. However, in the NTP studies, these other tumor types are seen primarily in rats (National Toxicology Program 2006a, 2006b).
In the 2006 NTP historical control database (consisting of data from over 1500 each of male and female F344/N rats and 1500 each of male and female B6C3F1 mice from all two-year studies within the most recent five years on the Toxicology Data Management System
Chemically induced nonneoplastic lesions of the lungs in rats and mice have not been well documented in the literature. In this manuscript we describe nonneoplastic lesions, which include acute to chronic inflammation and cellular infiltrates (most often histiocytic in type), as well as hemorrhage, fibrosis, congestion, and metaplasia.
Metastatic lesions are often observed in the lungs of rodents, and in the evaluated NTP studies, primary hepatocellular carcinoma was the most common metastatic lesion found in the lungs of mice, whereas in rats, skin was most often the primary site of origin of pulmonary metastatic lesions.
In this manuscript, we describe the histopathology of the most common neoplastic and nonneoplastic lesions induced by NTP-tested chemicals in the lungs of rats and mice and summarize metastatic lesions and NTP data related to chemically induced, site-specific pulmonary neoplasia.
MATERIALS AND METHODS
Pathology Data
Data were summarized from NTP Databases (http://ntpserver.niehs.nih.gov) and the 2006 NTP Historical Control Database (http://ntp-server.niehs.nih.gov). Representative images of pathologic lesions were obtained from VoxPort (NIEHS Management, NTP Archives Digital Images, 2007) or directly from the NTP archives. Within individual NTP studies from which pathology data were compiled, all animal procedures complied with the Public Health Service Policy on the Humane Care and Use of Animals. All tissue collection, processing, sectioning, and staining procedures were done in accordance with the Specifications for the Conduct of Studies to Evaluate the Toxic and Carcinogenic Potential of Chemical, Biological, and Physical Agents in Laboratory Animals for the National Toxicology Program (October 2006) (http://ntp-server.niehs .nih.gov) and are described briefly. The lungs were prepared for histologic evaluation by introducing 10% buffered formalin (approximately 1–2 mL for mice and 4–8 mL for rats) into the trachea until the lungs were completely filled to normal inspiratory volume. Next, the trachea was tied, and the organs were placed in formalin. An entire coronal section of both right and left lungs, including mainstem bronchi, was submitted for processing. Tissue sections were cut at 4–6 µm and stained with hematoxylin and eosin (H & E).
RESULTS
Chemicals and Routes of Exposure
To date, there are 545 peer-reviewed NTP reports of long-term carcinogenicity studies in rats and/or mice. Of these studies, sixty-six (12%) reported “clear,” “positive,” or “some” evidence of carcinogenicity for site-specific induction of lung tumors in rats and/or mice (Table 1). Nearly twice as many chemicals induced primary lung neoplasia in mice as in rats (Table 1). Of the studies associated with lung tumor induction, approximately 35% were inhalation and 35% were gavage studies, whereas chemical administration by dosed-feed, dosed-water, topical, intraperitoneal, or in utero routes accounted for 18%, 6%, 3%, 1%, and 1% of the studies, respectively (Table 2).
Pathology
Neoplastic Lesions
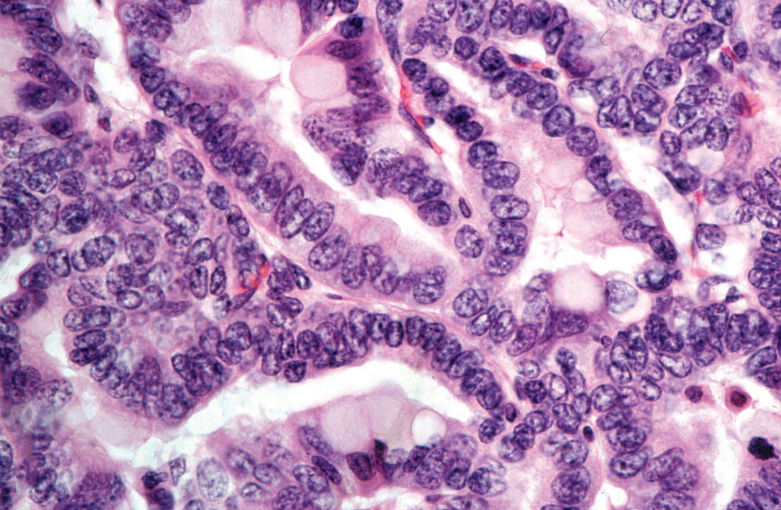
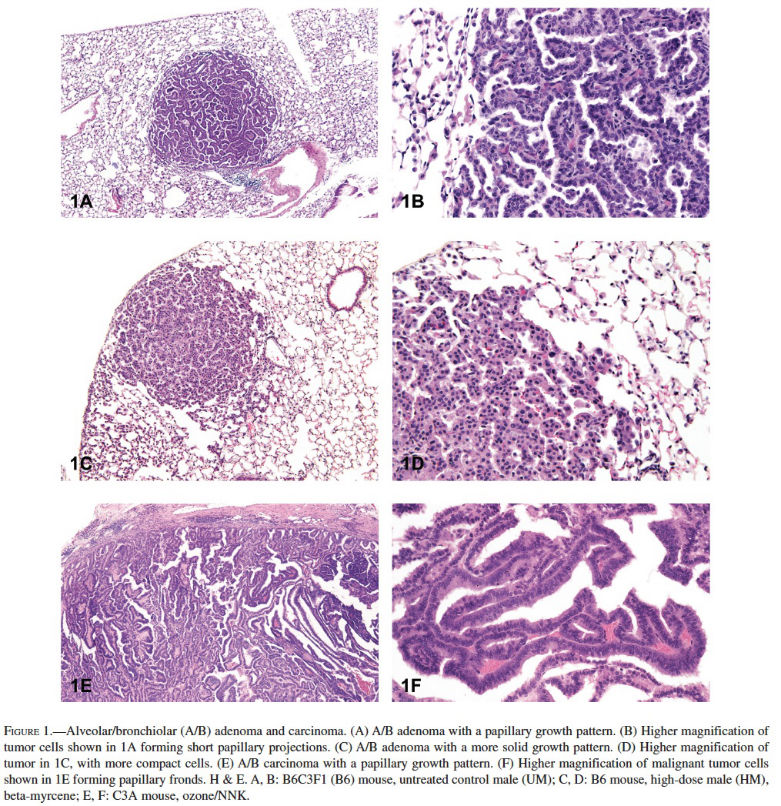
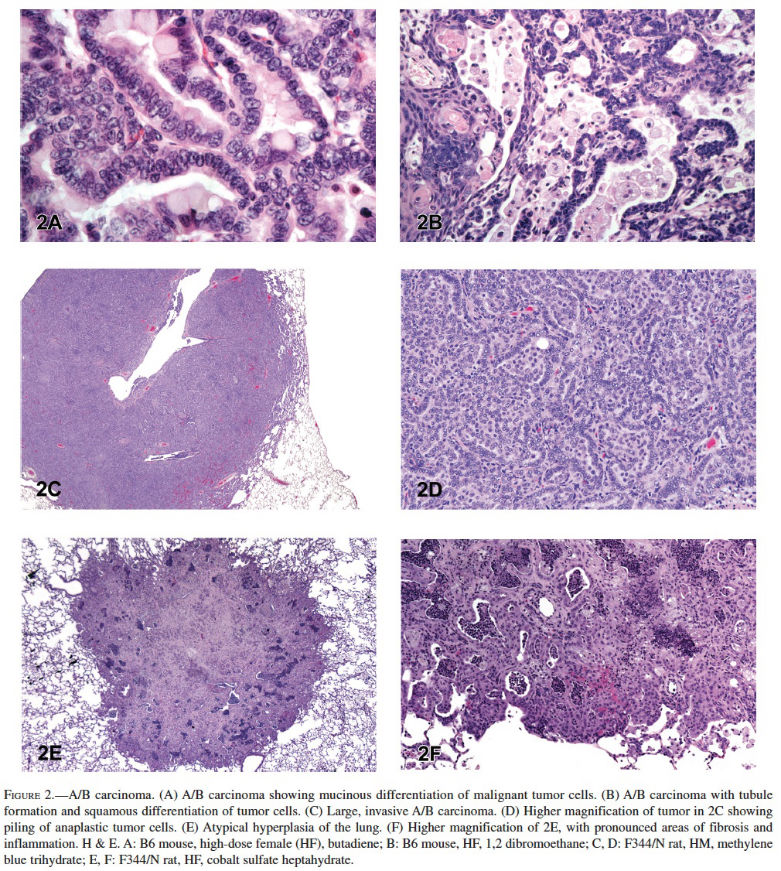
Alveolar/Bronchiolar Adenoma and Carcinoma: The most common chemically induced tumors in the lungs of rats and mice originated in the alveolobronchiolar regions of the lung and were classified as alveolar/bronchiolar (A/B) adenomas or carcinomas. A/B tumors accounted for 90% of the NTP studies reported to have primary lung tumors. Microscopically, A/B adenomas are composed of cuboidal cells that obliterate or efface the normal alveolar architecture (Figures 1A and 1B). The tumor cells tend to fill adjacent alveolar spaces, have little to no pleomorphism or atypia, and have low mitotic activity. A/B adenomas often compress the surrounding tissue; however, there is no invasion. Growth patterns are similar to those observed in spontaneous A/B adenomas and may be papillary, solid, or a combination thereof (Figures 1C and 1D). In contrast, A/B carcinomas are composed of cells that may be pleomorphic, ranging from low cuboidal to columnar, often lining fronds of connective tissue forming papillary structures that extend into alveolar spaces (Figures 1E and 1F). Frequently, they have variable growth patterns similar to spontaneous A/B carcinomas; however, induced carcinomas may show a greater tendency to differentiate into mucinous or squamous cell types and show tubular or glandular growth patterns (Figures 2A and 2B). The tumor cells frequently have a high nucleus–cytoplasm ratio, nuclear piling, or multilayered growth, cellular and nuclear atypia, and increased mitotic activity (Figures 2C and 2D). A/B carcinomas can invade the surrounding parenchyma and may metastasize within the lung or to extrapulmonary sites. Some A/B carcinomas have areas of necrosis, sterol clefts, fibrosis, and infiltrates of alveolar macrophages within the tumors and adjacent alveoli. These A/B carcinomas must be differentiated from atypical hyperplasia, which is characterized by fibrosis and dysplastic alveolar epithelium, which lacks sufficient atypia to be considered neoplastic. Atypical hyperplasia is often observed in the lungs of rats exposed repeatedly to particulates via inhalation (Figures 2E and 2F).
Cystic Keratinizing Epithelioma and Squamous Cell Carcinoma: Cystic keratinizing epithelioma is most often seen in rats exposed to particles such as diesel exhaust, carbon black, talc, quartz or titanium dioxide (Boorman et al. 1996; Mohr et al. 2006; Rittinghausen et al. 1997). At the NTP, these tumors were observed in Sprague-Dawley (SD) rats following exposure to polychlorinated biphenyls (PCBs), dioxin, and dioxin-like compounds (National Toxicology Program, 2006a, 2006b).
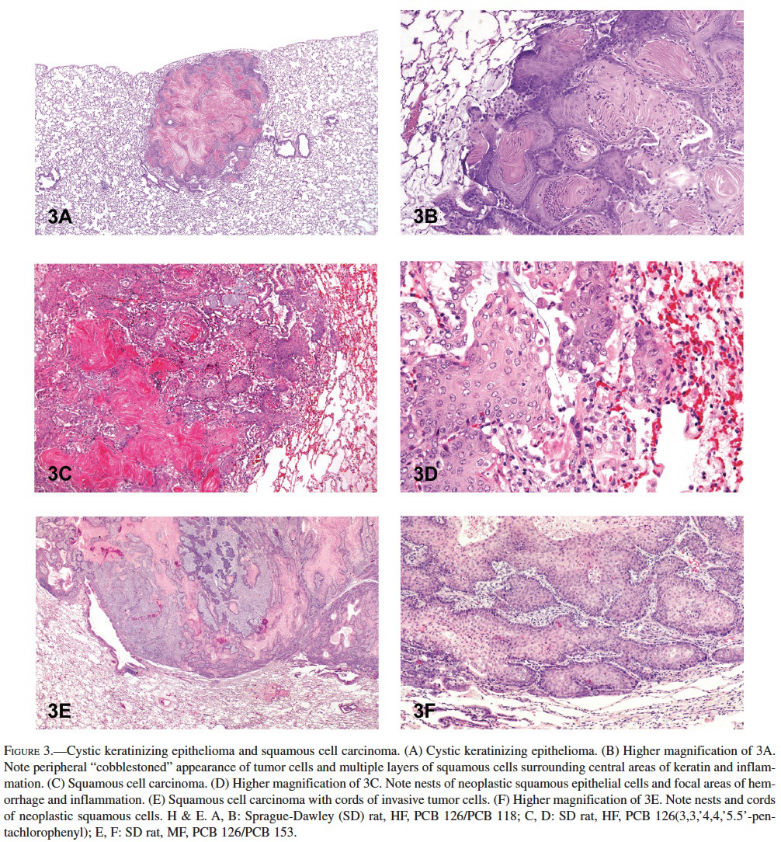
Cystic keratinizing epitheliomas in the lung tend to occur late in a study, are generally observed in rats in high-exposure groups, are much more common in females than males, and are rare in controls (Boorman et al. 1996). The historical control incidence of cystic keratinizing epithelioma in NTP B6C3F1 mice, F344/N rats, and Sprague-Dawley rats is zero (0/340 for SD rats), although it has been reported elsewhere to occur spontaneously in a Sprague-Dawley rat (Rittinghausen and Kaspareit 1998). Microscopically, the tumors consist of squamous cells that tend to form a multilayered wall that typically surrounds a central area of keratin, fibrosis, and/or inflammation (Figures 3A and 3B). Peripherally, the tumors have a cobblestoned appearance and expand into the adjacent alveoli.
In NTP studies, squamous cell carcinoma of the lung is rarely induced by chemical exposure; less than 10% of those studies with lung tumor induction led to a diagnosis of squamous cell carcinoma. Of those chemicals that induced squamous cell carcinoma in the lung, induction was in male or female rats, but not in mice. The historical control incidence of squamous cell carcinoma in B6C3F1 mice is zero, whereas for male and female F344/N rats the incidence is less than 0.1%. The NTP reserves the diagnosis of primary lung squamous cell carcinoma for those cases where greater than 90% of the tumor is composed of neoplastic squamous cells (Dixon and Maronpot 1991). In rats and mice, neoplasms with equal to or less than 90% squamous cells are classified as A/B carcinoma with squamous differentiation based on the assumption that they are variants of A/B carcinoma. Squamous cell carcinoma of the lung typically has the same histologic characteristics as those found in other organs such as the forestomach or skin. They often consist of infiltrative tumor cells associated with focal areas of necrosis, inflammation, and hemorrhage (Figures 3C–3F). They are typically composed of irregular nests or cords of invasive anaplastic epithelial cells that may show prominent intercellular bridges (Figure 3D). The tumor cells may show cellular atypia, mitotic activity, or dysplasia. They sometimes appear as individualized keratinized cells or surround “keratin” or “horn” pearls, which consist of concentric layers of keratinized squamous cells with keratin formation toward the center. Fibrosis can often be seen with squamous cell carcinoma of the lungs in rats.
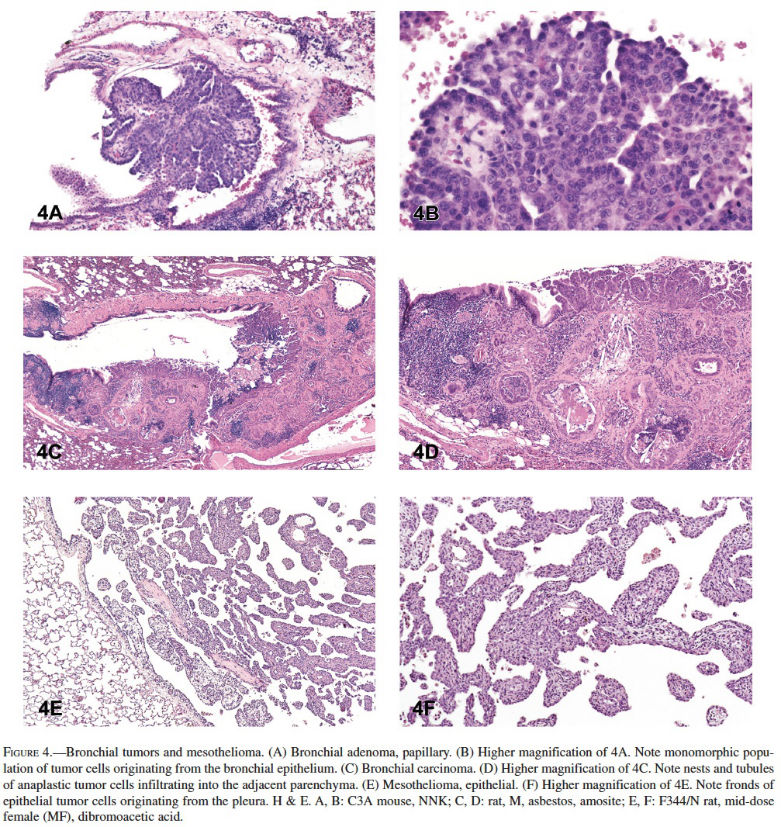
Bronchial Adenoma and Carcinoma: Spontaneous and induced bronchial adenoma and carcinoma are rare in rats and mice in contrast to humans, although they are occasionally observed in mice and rats after chemical exposure (Figures 4A–4D). The historical control incidence for bronchial tumors in rats and mice in NTP studies is zero. Bronchial neoplasms are diagnosed only when the origin of the proliferation is clearly the major airways. Microscopically, bronchial adenomas appear as polypoid or exophytic papillary growths that protrude into the airway lumen and originate from one or more sites of the bronchial mucosa (Figures 4A and 4B). The tumor cells are typically cuboidal to columnar and have round to oval centrally or basally located nuclei. The tumor cells are often monomorphic and well differentiated, and they show little or no nuclear or cytoplasmic pleomorphism, or nuclear atypia. Benign bronchial neoplasms do not invade the bronchial walls, whereas cells of bronchial carcinomas will commonly infiltrate the wall of bronchi. Bronchial carcinomas are characterized by papillary or tubular/glandular growths of anaplastic tumor cells that may partly extend into or fill the bronchial lumen (Figures 4C and 4D). These malignant tumors can be infiltrative, and tumor cells may range from cuboidal to tall columnar and often have cytologic and nuclear pleomorphism and nuclear atypia; foci of mucinous cell differentiation can occur.
Mesothelioma: In contrast to peritoneal mesotheliomas in rats, spontaneous primary mesothelioma of the lung pleura is rare in F344/N rats and B6C3F1 mice, and it is very rarely reported as an induced tumor. Proposed early mesotheliomas grow along the pleural surfaces and rarely infiltrate the lung. The tumors can be classified as epithelial (tubular, papillary, or solid) or mesenchymal (fibrous). The neoplastic epithelial cells in mesotheliomas are typically small and uniformly cuboidal and form glandular-like structures, line papillary fronds of fibrovascular tissue, or form solid sheets. Advanced or late mesotheliomas, on the other hand, can be infiltrative and consist of large atypical pleomorphic cells that may have glandular-like structures, solid areas, tubulopapillary, or mixed growth patterns (Figures 4E and 4F). Mesenchymal mesotheliomas must be distinguished from fibrosarcomas. Mesenchymal mesotheliomas are often composed of bundles of spindle to plump elongate, or pleomorphic cells within a fibrous matrix.
Mesenchymal Neoplasms
Spontaneous primary mesenchymal neoplasms of the lung are rare in mice and rats, and they are rarely induced by chemicals. Primary hemangiosarcoma, hemangioma, fibrosarcoma, fibroma, leiomyosarcoma, and sarcoma have been diagnosed in the lungs of rats and mice in NTP studies.
Metastatic and Systemic Neoplasms
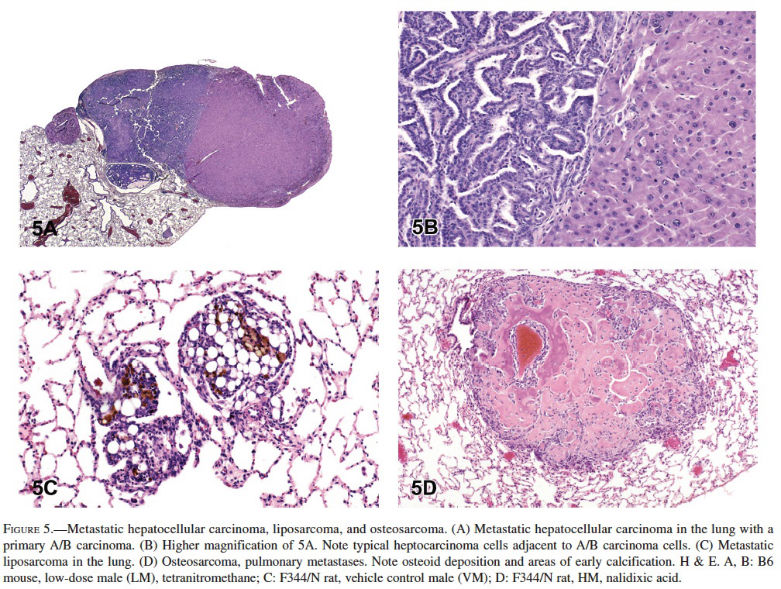
In mice, the liver (33.3%) was the most common primary site of origin of tumors metastatic to the lungs. Metastatic hepatocellular carcinoma, hepatoblastoma, histiocytic sarcoma, hepatocholangiocarcinoma, cholangiocarcinoma, hemangiosarcoma, and malignant fibrous histiocytoma were observed in the lungs of mice in NTP studies (Figures 5A and 5B). In mice, the skin was the second most common primary site of origin of tumors metastatic to the lungs (15.8%), and it was the most common site of origin of metastatic tumors in rats (16.0%). Metastatic sarcoma, fibrosarcoma, liposarcoma, malignant fibrous histiocytoma, and squamous cell carcinoma originating from the skin were diagnosed as metastatic tumors in the lungs of rats and mice in the NTP studies (Figure 5C). In rats, bone (11.9%) was the second most common primary site of metastatic tumors, with osteosarcoma being most often diagnosed (Figures 5D). Other metastatic tumors observed in the lungs of rats and mice included chordoma, Zymbal’s gland carcinoma, Harderian gland adenocarcinoma, and mammary adenocarcinoma.
Of the spontaneous systemic tumors that may involve the lung, mononuclear cell leukemia is the most common in F344/N rats (male = 41.6%; female = 26.8%). Malignant lymphoma is often observed systemically in control female B6C3F1 mice (19.2%; male = 4.1%), with an incidence of less than 1% in male and female F344/N rats. Histiocytic sarcoma is more often observed in female B6C3F1 mice (1.7%), and the incidence in male B6C3F1 mice and male and female F344/N rats is less than 1.0%.
Nonneoplastic Lesions
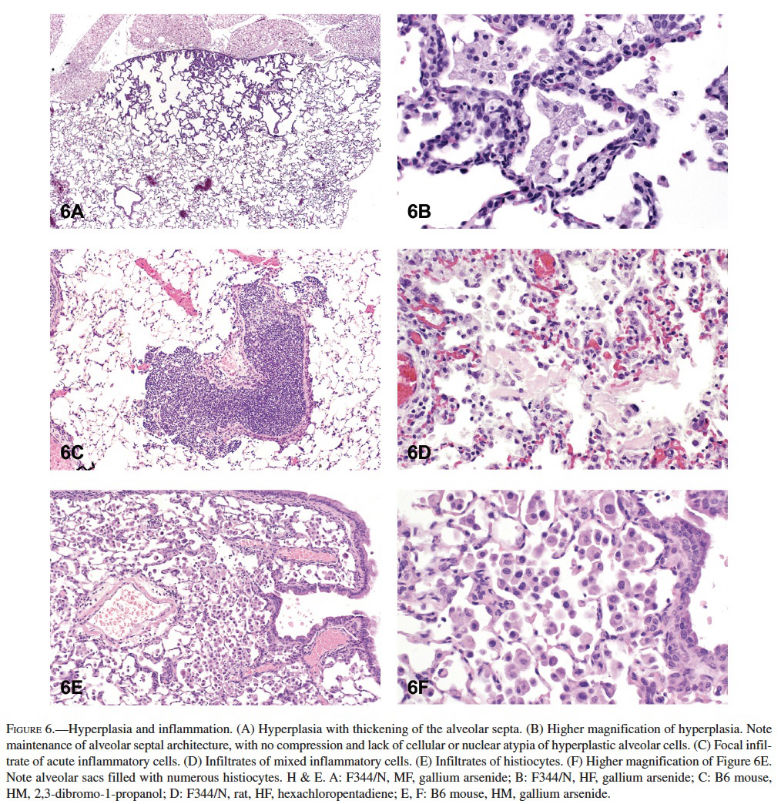
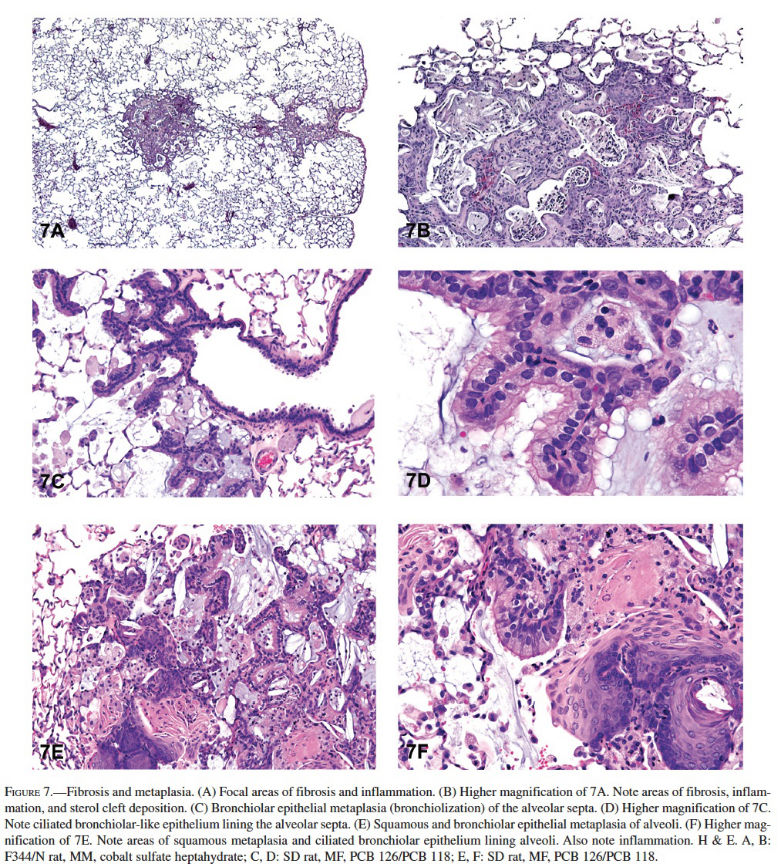
Hyperplasia was the most common nonneoplastic lesion observed in the lungs of mice (Table 3). Inflammation and hyperplasia were equally the most commonly diagnosed nonneoplastic lesions in rats (Table 3). Hyperplasia of the alveolar septal epithelium is typically characterized as focal and poorly demarcated, but it does not result in compression of the surrounding parenchyma. Proliferation of alveolar type II epithelial cells often results in thickening of the alveolar septa; however, the alveolar septal architecture is maintained (Figures 6A and 6B). The cells generally lack cellular or nuclear atypia, and mitoses are uncommon. Induced bronchiolar hyperplasia is rare in rats and mice, although it can occur. Inflammation was one of the most common lesions observed in the lungs of rats, and the second most common lesion in mice in NTP studies. Inflammation was most frequently characterized as suppurative, chronic, chronic-active, granulomatous, or necrotizing (Figures 6C and 6D). Cellular infiltrates, most frequently consisting of histiocytes, were diagnosed in the lungs of both rats and mice; in addition, nonneoplastic changes such as hemorrhage and congestion were observed (Figures 6E and 6F). Mineralization, squamous metaplasia of the alveolar septa, and fibrosis were more commonly observed in rats than in mice (Figures 7A and 7B). Other nonneoplastic changes such as crystal (sterol/cholesterol cleft) deposition, bronchiolar metaplasia (bronchiolization) of the alveolar epithelia, alveolar proteinosis, and keratin cysts were also reported in treated rats and mice exposed to chemicals in studies at the NTP (Figures 7C and 7D). Multiple nonneoplastic lesions were often diagnosed in the lung of a single animal (i.e., inflammation and squamous metaplasia; inflammation, squamous metaplasia, and fibrosis) (Figures 7E and 7F). Thrombosis was often observed in mice, but not in rats (Table 3).
SUMMARY
The most frequently induced tumors in the lungs were alveolar/bronchiolar (A/B) adenoma and/or carcinoma in rats and mice. Of the sixty-six reports that showed evidence of site-specific carcinogenicity in the lung, sixty reports indicated A/B adenoma, A/B carcinoma, or both as the primary tumors induced. The six exceptions were PCBs, 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD), or dioxin mixtures studies in female SD rats, in which primary cystic keratinizing epitheliomas of the lung were induced (National Toxicology Program 2006a, 2006b). Cystic keratinizing epitheliomas are often observed in the lungs of rats following exposure to particles such as diesel exhaust, black carbon, quartz, talc, and nickel oxide (Boorman et al. 1996; Mohr et al. 2006; Rittinghausen and Kaspareit 1998). Other compounds such as nickel (subsulfide and oxide), cobalt sulfate heptahydrate, dimethyl hydrogen phosphite, indium phosphide and tetranitromethane all induced squamous cell carcinoma in the lungs of rats in addition to A/B tumors. Sarcomas and mixed malignant tumors were observed in rats exposed to tetranitromethane. Induced bronchial adenoma and carcinoma and mesotheliomas were rarely observed in the lungs of rats and mice exposed to NTP chemicals. In NTP studies, mesenchymal tumors of the lung were also less often diagnosed in chemically treated rats and mice. When present, these included hemangioma, hemangiosarcoma, fibroma, fibrosarcoma, leiomyosarcoma, and sarcoma. Among the spontaneous systemic tumors affecting the lung, mononuclear cell leukemia was the most common systemic tumor found in F344/N rats (male = 41.6%; female = 26.8%). Spontaneous malignant lymphoma was often observed systemically in female B6C3F1 mice (19.2%; male = 4.1%), with an incidence of less than 1% in male and female F344/N rats. Spontaneous histiocytic sarcoma was less often observed in male and female B6C3F1 and F344/N rats, with the incidence being less than 2.0%.
The liver was the most common primary site of origin for metastatic lung tumors in mice, whereas in rats, skin was most often the primary site of origin. Metastatic hepatocellular carcinoma, hepatoblastoma, histiocytic sarcoma, hepatocholangiocarcinoma, cholangiocarcinoma, hemangiosarcoma, and malignant fibrous histiocytoma were observed in the lungs of mice from NTP studies. Metastatic sarcoma, fibrosarcoma, malignant fibrous histiocytoma, and squamous cell carcinoma originating from the skin were often reported in the lungs of rats. Similar metastatic lesions were also found in mice, because the skin was the second most common site of origin. Bone was the second most common primary site of origin of metastatic lesions to the lungs of rats.
The most common nonneoplastic lesions in the lungs of rats and mice were hyperplasia of the alveolar epithelium and inflammation. Most inflammatory lesions were categorized as acute, acute/chronic, or chronic; suppurative, granulomatous, and other types were less frequently observed in the lungs of rats and mice from NTP studies. Cellular infiltrates of primarily histiocytes were commonly observed in the alveoli of both rats and mice. Hemorrhage and congestion were common changes observed in both species. Squamous metaplasia of the alveoli, mineralization, and fibrosis occurred more often in rats than in mice. Thrombosis was observed in mice but was uncommon in rats. It should be noted that it is often difficult to differentiate nonneoplatic lesions such as A/B hyperplasia and histiocytic infiltrates induced in some of the inhalation studies from similar but spontaneous lesions related to aging, especially in rats.
In conclusion, A/B adenomas and carcinomas are the most frequently diagnosed chemically induced tumors in the lungs of rats and mice in NTP studies, with hyperplasia and inflammation being the most common nonneoplastic changes in both species. The lung is the second most common target site of tumor induction of chemicals tested in the NTP two-year rodent bioassay. Of the 545 peer-reviewed NTP studies to date, sixty-six produced significant site-specific neoplasia in the lung of rats and mice. Primary lung tumor diagnoses are most often associated with chemicals administered by the inhalation, gavage, or dosed-feed routes, with all other routes of administration composing a total of 11%.
ACKNOWLEDGMENTS
The authors thank Drs. John Peckham and Abraham Nyska for their critical review of this manuscript. This work was supported by the Intramural Research Program of the National Institutes of Health (NIH) and the National Toxicology Program (NTP), National Institute of Environmental Health Sciences.
REFERENCES
Belinsky, S. A., Devereux, T. R., White, C. M., Foley, J. F., Maronpot, R. R., and Anderson, M. W. (1991). Role of Clara cells and type II cells in the development of pulmonary tumors in rats and mice following exposure to a tobacco-specific nitrosamine. Exp Lung Res 17, 263–78.
Boorman, G. A., Brockmann, M., Carlton, W. W., Davis, J. M., Dungworth, D. L., Hahn, F. F., et al. (1996). Classification of cystic keratinizing squamous lesions of the rat lung: report of a workshop. Toxicol Pathol 24, 564–72.
Brooks, D. R., Austin, J. H., Heelan, R. T., Ginsberg, M. S., Shin, V., Olson, S. H., et al. (2005). Influence of type of cigarette on peripheral versus central lung cancer. Cancer Epidemiol Biomarkers Prev 14, 576–81.
Dixon, D., and Maronpot, R. R. (1991). Histomorphologic features of spontaneous and chemically-induced pulmonary neoplasms in B6C3F1 mice and Fischer 344 rats. Toxicol Pathol 19, 540–56.
Herbert, R. A., Stegelmeier, B. S., Gillett, N. A., Rebar, A. H., Carlton, W. W., Singh, G., et al. (1994). Plutonium-induced proliferative lesions and pulmonary epithelial neoplasms in the rat: immunohistochemical and ultrastructural evidence for their origin from type II pneumocytes. Vet Pathol 31, 366–74.
Kauffman, S. L. (1981). Histogenesis of the papillary Clara cell adenoma. Am J Pathol 103, 174–80.
Kauffman, S. L., Alexander, L., and Sass, L. (1979). Histologic and ultrastructural features of the Clara cell adenoma of the mouse lung. Lab Invest 40, 708–16.
Mohr, U., Ernst, H., Roller, M., and Pott, F. (2006). Pulmonary tumor types induced in Wistar rats of the so-called “19-dust study.” Exp Toxicol Pathol 58, 13–20.
National Toxicology Program (2006a). Toxicology and carcinogenesis studies of 3,3,’4,4,’5-pentachlorobiphenyl (PCB 126) (CAS No. 57465–28–8) in female Harlan Sprague-Dawley rats (Gavage Studies). NTP TR 520, NIH Publication No. 06–4454, NIEHS, Research Triangle Park, NC. National Toxicology Program (2006b). Toxicology and carcinogenesis studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (CAS No. 1746–01–6) in female Harlan Sprague-Dawley rats (Gavage Studies). NTP TR 521, NIH Publication No. 06–4468, NIEHS, Research Triangle Park, NC.
Nikitin, A. Y., Alcaraz, A., Anver, M. R., Bronson, R. T., Cardiff, R. D., Dixon, D., et al. (2004). Classification of proliferative pulmonary lesions of the mouse: recommendations of the mouse models of human cancers consortium. Cancer Res 64, 2307–16.
Ohshima, M., Ward, J. M., Singh, G., and Katyal, S. L. (1985). Immunocytochemical and morphological evidence for the origin of N-nitrosomethylurea-induced and naturally occurring primary lung tumors in F344/NCr rats. Cancer Res 45, 2785–92.
Parsa, I., and Kauffman, S. L. (1983). Malignant Clara cell line derived from ethylnitrosourea-induced murine lung adenomas. Cancer Lett 18, 311–16.
Rehm, S., Takahashi, M., Ward, J. M., Singh, G., Katyal, S. L., and Henneman, J. R. (1989). Immunohistochemical demonstration of Clara cell antigen in lung tumors of bronchiolar origin induced by N-nitrosodiethylamine in Syrian golden hamsters. Am J Pathol 134, 79–87.
Rittinghausen, S., and Kaspareit, J. (1998). Spontaneous cystic keratinizing epithelioma in the lung of a Sprague-Dawley rat. Toxicol Pathol 26, 298–300.
Rittinghausen, S., Mohr, U., and Dungworth, D. L. (1997). Pulmonary cystic keratinizing squamous cell lesions of rats after inhalation/instillation of different particles. Exp Toxicol Pathol 49, 433–46.
Sato, T., and Kauffman, S. L. (1980). A scanning electron microscopic study of the Type II and Clara cell adenoma of the mouse lung. Lab Invest 43, 28–36.
Stellman, S. D., Muscat, J. E., Hoffmann, D., and Wynder, E. L. (1997). Impact of filter cigarette smoking on lung cancer histology. Prev Med 26, 451–56.
Ward, J. M., Singh, G., Katyal, S. L., Anderson, L. M., and Kovatch, R. M. (1985). Immunocytochemical localization of the surfactant apoprotein and Clara cell antigen in chemically induced and naturally occurring pulmonary neoplasms of mice. Am J Pathol 118, 493–99.
Wynder, E. L., and Muscat, J. E. (1995). The changing epidemiology of smoking and lung cancer histology. Environ Health Perspect 103 (Suppl 8), 143–48.