Acrylamide is an important chemical with widespread industrial and other uses in addition to generalized population exposure from certain cooked foods. Previous rat studies to assess the carcinogenic potential of acrylamide have been carried out exclusively in the Fischer 344 rat with identification of a number of tumors amongst which mesotheliomas of the tunica vaginalis is an important tumor endpoint in the classification of acrylamide as a ‘probably human carcinogen. In a rat carcinogenicity study to determine the human relevance of mesotheliomas Wistar Han rats were exposed to 0, 0.5, 1.5, or 3.0 mg acrylamide/kg body weight/day in drinking water starting at gestation day 6. At the end of two years, mammary gland fibroadenomas in females and thyroid follicular cell tumors in both sexes were the only tumors increased in acrylamide treated rats. These tumor endpoints have rat-specific modes of action suggesting less likelihood of human cancer risk than previously estimated. This study demonstrates that tunica vaginalis mesotheliomas are strain specific and not likely of genotoxic origin.
Keywords
Mammary fibroadenomas; Thyroid follicular cell tumors; In utero exposure; Neuropathy; Myopathy; Carcinogenicity
1. Introduction
Acrylamide (ACR) is a monomer used in the manufacture of polymers for mining, oil and natural gas processing, paper production, waste processing, as well as hospital, laboratory and other uses. Adverse health effects from industrial emissions and exposure has been extensively studied Lipworth et al., 2012) and no adverse effects have been reported with daily exposure up to 2.1 mg/kg/day (Erdreich and Friedman, 2004). Nevertheless, due to various risk assessments, several of the workplace permissible exposure limits (PEL) are under evaluation and may be lowered considerably.
In addition to industrial exposure to acrylamide, generalized population exposure to acrylamide in foodstuffs has been documented (Vesper et al., 2008 and Vesper et al., 2010). This exposure to ACR has become a worldwide concern because of its generation in a variety of carbohydrate rich foods when cooked at temperatures exceeding 120 °C (Mottram et al., 2002 and Friedman, 2003). At these temperatures, Maillard reaction of sugars with asparagine residues produce acrylamide (Friedman, 2005). The 64th Joint FAO/WHO Expert Committee on Food Additives concluded that an intake of 1 μg/kg body weight/day of ACR could be taken to represent the average for the general population (JECFA, 2005). The USFDA estimated a mean intake of 0.4 μg/day for ages 2 and up with a 90% confidence limit of >2 μg/kg (http://www.fda.gov/downloads/Food/FoodborneIllnessContaminants/UCM197239.pdf). This equates to an adult male exposure of 1.4 μg/day.
Acrylamide has previously been shown to cause fibroadenomas of the mammary gland and thyroid gland follicular tumors in rats (Johnson et al., 1986, Friedman et al., 1995 and Beland et al., 2013). Since these tumor sites have well documented rat-specific modes of action (Neumann, 1991, Ben-Jonathan et al., 2008 and Alison et al., 1994), a primary consideration in classification of acrylamide as a “probable human carcinogen” centers on the induction of tumors of the tunica vaginalis mesotheliomas (TVMs) in F344 rats (Johnson et al., 1986, Friedman et al., 1995 and Beland et al., 2013). These unique mesotheliomas in acrylamide-treated F344 rats might be considered a potential human health risk if they were caused by a genotoxic mechanism (Wall, 2005). However, F344 rats have an elevated incidence of TVMs secondary to their known high spontaneous incidence of Leydig cell tumors (Maronpot et al., 2009). TVMs found in the acrylamide studies have the same biological behavior, ultrastructure, and morphology as TVMs found in control rats, prompting the conclusion that acrylamide accelerates the appearance of these background tumors (Damjanov and Friedman, 1998 and Maronpot et al., 2009). It has also been shown that the time-to-tumor and incidence data for TVMs are consistent with a non-genotoxic mechanism (Maronpot et al., 2009). The mechanism of action of acrylamide induction of TVMs has been elaborated (Shipp et al., 2006).
The EPA calculation of the cancer risk for ACR relies on 2 important considerations. The first is that acrylamide acts as a mutagen. We will not discuss this assumption here. Second, that tunica vaginalis mesotheliomas (TVMs) are added to thyroid tumors in males (EPA, 2010). Depending on which study is used (Johnson, Friedman, or Beland) there are either twice as many TVMs as thyroid tumors or the same number. While the biochemistry supports an endocrine mechanism for TVMs, there are no data directly demonstrating this mechanism (EPA, 2010). In order to clarify the mode of action of acrylamide induced neoplasms, we sought out a strain of rats which did not get Leydig cell tumors and had a low level of background TVMs to examine a potential endocrine mode of action. If acrylamide acted by a genotoxic mode of action, it would necessarily produce TVMs in a second strain. Wistar Han rats were selected due to their longevity and low incidence of Leydig cell tumors. We report here the oncogenic response of Wistar Han rats to orally administered acrylamide. Furthermore, concern has been expressed that children might be at higher risk to acrylamide due to greater dietary acrylamide intake (EPA Science Advisory Board, 2008). This parameter was evaluated in the current research by initiating dosing on gestation day 6 in pregnant rats and continued to termination of the F1 offspring 2 years later.
2. Materials and methods
2.1. Study conduct
This study was conducted under GLP guidelines enacted in Germany in the ‘Chemikaliengesetz’, current edition and OECD Principles of Good Laboratory Practice’ Document Nos. 1, 8 and 13 ENV/MC/CHEM (98)17, ENV/JM/MONO(99)24, and ENV/JM/MONO (2002)9 and was externally audited. The following guidelines were considered: OECD Guideline for the Testing of Chemicals No. 453: Combined chronic toxicity/carcinogenicity studies, adopted 7 September 2009; and EC method 8.33. Combined chronic toxicity/carcinogenicity test, 88/303/EEC; Official Journal of European Communities, L 133 1988.
2.2. Test article
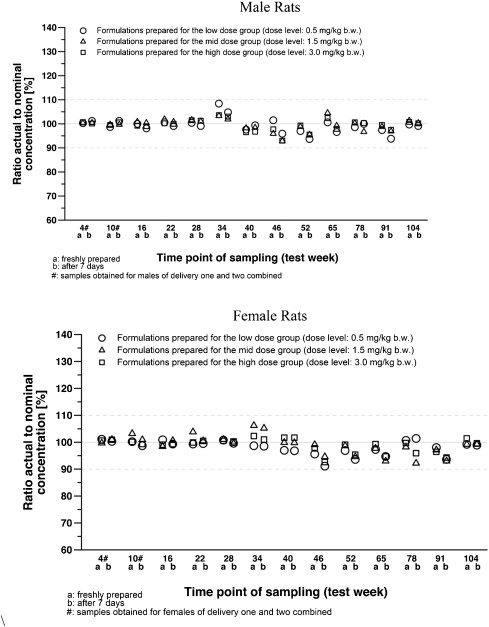
Acrylamide (C3H3NO, CAS no 79-06-1, 1,2-propenamide; >99.9% pure) was purchased from Sigma Aldrich as a white, odorless, crystalline solid which is stable at room temperature for at least 2 years. Acrylamide was administered daily in drinking water at the following doses: 0, 0.5, 1.5 and 3.0 mg/kg/day. Water was available ad libitum. In a separate study, solutions of acrylamide were prepared in tap water and evaluated for stability at room temperature at 6, 13, 20, 27, 41, 55 or 90 test days after preparation and recovery ranged from 96.9% to 102.6%. In the present study acrylamide solutions were prepared weekly and after concentration adjustment for body weight, water bottles were changed weekly. Aliquots for analysis were taken at the beginning and end of exposure to determine stability. Acrylamide concentration in the drinking water was determined at test week 4, 10, 16, 22, 28, 34, 40, 46, 52, 65, 78, and 91. Fig. 1 shows that acrylamide was stable in the animal cages for the weeks of exposure.
2.3. Animals
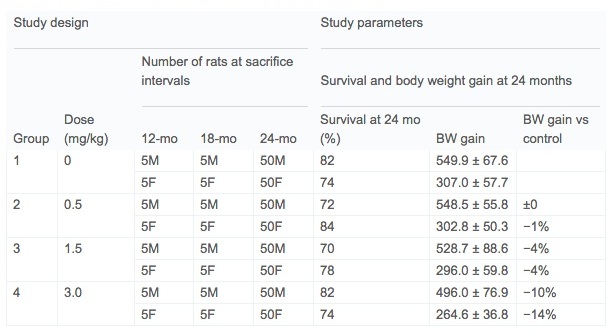
Sperm positive female Wistar Han™/RccHan™:WIST rats were obtained from Harlan Laboratories GmbH, Serumweg 48, 27324 Eystrup, Germany in multiple deliveries. At gestation day 6 pregnant dams were provided acrylamide in their drinking water with exposures continuing in F1 offspring through postnatal day (pnd) 722. Rats were housed 1 per cage in MACROLON cages with granulated wood bedding (Brandenburg, 49424 Goldenstedt/Arkeburg). Study design is summarized in Table 1. Sixty male and sixty female rats were selected for the chronic study as well as 5 sentinel animals. Five rats per sex per group were sacrificed at 12 months and 18 months. Animal rooms were alternately lit (about 150 lx at approximately 1.50 m room height) and darkened in a 12-h lighting cycle. Cage side observations were conducted twice per day during the week and once per day on weekends. Ophthalmological and auditory examinations were conduct at the interim kills (12 and 18 months) and at termination.
On day 4 after birth, the weights of the pups were determined. The size of each litter was adjusted by eliminating extra pups to yield, as nearly as possible, five males and five females per litter. The remaining animals were allowed to remain with the dams until day 21 of lactation (weaning). On lactation day 21, the F1 animals were randomized using a computer randomization program to assign the animals to the subsets within each group.
2.4. Assessment of physical and functional development
Neurological determinations were conducted on 5 rats per sex per group. Ear opening, eye opening, cleavage of the balanopreputial gland, vaginal opening and upper incisor eruption were assessed. No significant changes were seen in any of these parameters. Functional tests that included open field behavior and passive avoidance (learning) were conducted at pnd 27 and passive avoidance (learning) at pnd 34. Functional tests included grip strength and locomotor activity. No changes were seen in these parameters.
2.5. Gross necropsy
After 52, 78 or 104 test weeks the respective animals were sacrificed under ether anesthesia by cutting the abdominal aorta, exsanguinated, weighed, and necropsied under the direction of a pathologist. For rats that died or were sacrificed prematurely, necropsy examinations were performed immediately after the animals were found dead or after sacrifice. All superficial tissues were examined visually and by palpation and the cranial roof removed to allow observation of the brain, pituitary gland and cranial nerves. After a ventral midline incision and skin reflection, all subcutaneous tissues were examined. The condition of the thoracic and abdominal viscera was noted with due attention to the thymus, lymph nodes and heart.
The weights of the following organs of all animals assigned for interim dissection and the first 10 surviving main study animals/sex/group of the main dissection were determined before fixation: adrenal gland (2), brain, epididymis (2), heart, kidney (2), liver, ovary (2), spleen, testis (2), thyroid including parathyroid (1), and uterus. Adrenals, gonads and kidneys were weighed individually and identified as left or right. Organ weights of rats that died or were sacrificed prematurely were recorded but not included into the mean value comparison.
2.6. Histopathological examination
The following organs of all animals (including deceased or sacrificed animals) were preserved in 7% buffered formalin: adrenal (2), aorta abdominalis, bone (os femoris with joint), bone marrow (os femoris, sternum), brain (cerebrum, cerebellum, medulla/pons), caecum, clitoral gland (2), coagulating gland with seminal vesicle, epididymis (2), extraorbital lacrimal gland (2), eye with optic nerve and Harderian gland (2), heart (left and right ventricles, septum), intestine, small (duodenum, jejunum, ileum—Swiss roll method), intestine, large (colon, rectum), kidney (2) and ureter, all macroscopically visible lesions, liver, lungs (with mainstem bronchi and bronchioles), lymph node (1, cervical), lymph node (1, mesenteric), mammary gland, muscle (skeletal, leg), nasal cavity with nasopharynx, nerve (sciatic), esophagus, ovary (2), pancreas, pituitary, preputial gland (2), prostate, salivary glands (mandibular, sublingual and parotid gland), seminal vesicle, skin (left flank), spinal cord (3 sections), spleen, sternum, stomach (forestomach and glandular stomach), testis (2), thymus, thyroid (2, incl. parathyroids), tissue masses or tumors (incl. regional lymph nodes), tongue (incl. base), trachea (incl. larynx), urinary bladder, uterus (incl. cervix and oviducts), vagina, and Zymbal’s gland. Parathyroids cannot always be identified macroscopically. The eyes were preserved in Davidson’s and the testes in Bouin’s solution for optimum fixation. Other tissues were fixed in 7% buffered formalin and stained with hematoxylin and eosin (H&E) or other appropriate stains for preparation of microscopic slides. All slides from control and high dose rats were evaluated as well as rats that died prematurely.
2.7. Statistics
The following statistical methods were used: χ2 test, Dunnett’s test, t-test Fisher exact test, and Peto analysis. These statistical procedures were used for all data.
3. Results
3.1. Clinical signs (F0 dams) and morphological landmarks (F1 pups)
For F0 generation dams no test article-related clinical signs or changes in body weight, food consumption, drinking water consumption or reproductive parameters from implantation until weaning were present. No litter values, body weight changes between groups, or test article-related physical development alterations in the F1 pups were present during the three-week lactation period. Neurological screening and post-natal functional development 21 days after birth were similar among all groups.
3.2. Main study mortality
No test article-related deaths were present in rats prior to test week 78. By the end of the study at 104 weeks, there was excellent survival ranging between 70 and 84% with acceptable body weight gain and no test article-related effect on mortality/survival among any of the treated groups and the controls (Table 1). Factors contributory to death or premature sacrifice were either of age-related origin (e.g. chronic renal disease, thrombo-endocarditis) and/or a result of poor clinical condition in tumor-bearing (e.g. pituitary adenoma of pars distalis, various soft tissue tumors) animals. There were no factors contributory to death that could be related to treatment with the test article. Interestingly, while there was no effect on longevity, there was a statistically significant decreased in mean survival in high dose (3.0 mg/kg) females (80.4 weeks) versus controls (94.5 weeks) specifically among decedents.
3.3. Main study parameters
Hind limb paralysis was present in 12 of 50 high dose (3.0 mg/kg) male rats starting at test week 79. Hind limb paralysis was not present in females. Body weight was reduced by up to 11% in high-dose males and up to 13% in high-dose females starting between 83 and 86 weeks on study with slightly reduced body weight and body weight gain at study termination. No test article-related changes in food or water consumption, ophthalmological findings, or auditory changes were present in main study rats. Calculation of acrylamide intake via drinking water confirmed that achieved dose levels were obtained and test article analyses were within 91 and 106% of nominal concentrations during the course of the study.
3.4. Macroscopic post mortem findings and organ weights
The only treatment-related macroscopic change was an increase in mammary gland enlargements in 21 of 50 high dose females versus 12 of 50 in control females. No test article-related changes in absolute or relative organ weights were present at the interim or final dissections.
3.5. Histopathology
Non-neoplastic lesions related to treatment were present only in rats at the terminal 104-week dissection. At this interval adrenal cortical vacuolation was present in high-dose (3.0 mg/kg) females (Table 2). Adrenal cortical vacuolation was represented by a discrete focal change comprised of enlarged, vacuolated cells with a mixture of clear and pale pink wispy cytoplasm and a centrally located condensed nucleus. Adrenal cortical vacuolation is an age-associated change common in rats (Laast et al., 2014). Some vacuolated focal lesions were associated with a few hypertrophied cells with homogeneous pink cytoplasm within the same focal lesion. Hemorrhage was present in occasional focal vacuolated lesions.
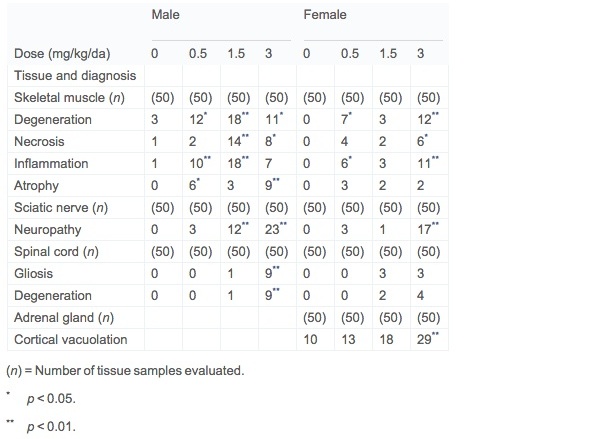
Table 2. Non-neoplastic lesions in target tissues in Wistar Han rat 2-year dose–water carcinogenicity study of acrylamide.
Hind limb skeletal muscle myopathy, sciatic nerve neuropathy, and spinal cord degeneration were present in both sexes (Table 2). Histopathological findings in hind limb skeletal muscle are consistent with a basic process of segmental muscle fiber degeneration with progression to necrosis and accompanied by repair. A constellation of changes including degeneration, necrosis, mixed inflammatory cell response to degeneration and necrosis, and repair identified by increased sarcolemma nuclei was identified in most cases. Muscle fiber atrophy and interstitial fibrosis was present in only a few cases.
The increased incidence of neuropathy was present in sciatic nerves from groups 3 and 4 males and group 4 females. Changes in affected rats consisted of axonal degeneration with localized fragmentation of neurofibers, loss of myelin, and inflammatory cell infiltrates, occasionally accompanied by cholesterol clefts and giant cells. Spinal cord vacuolar degeneration and/or increased cell density representative of gliosis was present in 18% of group 4 males and at 4 to 8% in groups 3 and 4 females, respectively. These changes were minimal and present in the dorsal column of one of three spinal cord cross sections.
A variety of other non-neoplastic spontaneous lesions typical for Wistar Han rats were equally present among treated and control groups.
Treatment-related neoplastic lesions were present in female mammary glands and in male and female thyroid glands in the present study (Table 3). Mammary fibroadenomas were increased in group 3 (12/50) and group 4 (15/50) females versus control (6/50) females attaining statistical significance (p = 0.0479) for group 4. The majority of the fibroadenomas were present in rats surviving to the 24-month terminal dissection. For the terminal sacrificed animals specifically this was 11/37 in group 4 versus 5/37 in group 1, not attaining statistical significance for this observation.
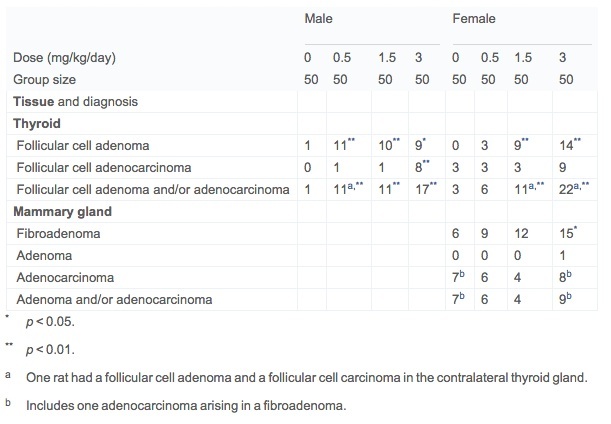
Table 2. Non-neoplastic lesions in target tissues in Wistar Han rat 2-year dose–water carcinogenicity study of acrylamide.
A dose-related increase in thyroid follicular cell neoplasms was present in all dosed male groups and in the mid and high dose female groups (Table 3). The majority of the neoplasms were follicular cell adenomas with a few follicular cell adenocarcinomas in the high dose males and females. With only a very few exceptions, these neoplasms were present at the terminal 24-month dissection interval. Follicular cell hyperplasia was equally present at a low frequency in all groups. The morphological features of both follicular cell adenomas and adenocarcinomas were typical of previously published reports (Rosol et al., 2013, Johnson et al., 1986, Friedman et al., 1995 and Wall, 2005). A spectrum of other tumors reflective of spontaneous background responses in chronic rat studies was observed in the present study.
While in each of the previous studies in Fischer 344 rats, mesotheliomas of the tunica vaginalis testes were observed, none were seen in this study (Table 4). Similarly, there was only one Leydig cell tumor in the testes. This is clearly a strain difference between the Fischer 344 rat and the Wistar Han rat. Similarly, other possible treatment-related neoplasia identified in previous Fischer 344 rat carcinogenicity studies, including clitoral gland carcinomas, cardiac schwannomas, islet cell adenomas, and oral cavity papillomas and carcinomas did not occur in the present Wistar Han study.
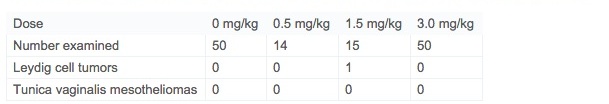
Table 4. Histological observations on testes and epididymides of Wistar Han rats in a 2-year carcinogenicity study.
4. Discussion
In contrast to prior chronic rat toxicity and carcinogenicity studies with acrylamide, this study included in utero exposure, use of a Wistar Han rat stock, and included a high dose of 3.0 mg/kg/day in both sexes. There was excellent survival in all groups with no treatment-related toxicity present at a 12- and 18-month interim dissection. Hind limb weaknesses in high dose males after the 18-month dissection and decreased body weight gain in both sexes at the high dose are clinical indications of having achieved a suitably high dose.
Non-neoplastic changes including sciatic nerve nephropathy, spinal cord degeneration, and hind limb myopathy have been reported previously in acrylamide studies (Burek et al., 1980 and Shipp et al., 2006) and were expected findings in the present study. Adrenal cortical vacuolation represents a common age-associated change in rats (Laast et al., 2014) and appears to have been exacerbated in the high dose females in the present study.
Focal adrenal cortical vacuolation was increased in high dose female rats in the present study. Adrenal cortical vacuolation is commonly seen in aged rats and is a focal degenerative change most frequently seen in the zona fasciculata (Laast et al., 2014). It occurred late in this study, being seen primarily in the 24-month terminal dissection rats. There was no evidence of other progressive degenerative cortical lesions in rats with focal adrenal cortical vacuolation. It has been diagnosed as lipoid hyperplasia (Greaves, 2012) and may represent a residual change following focal adrenal cortical hyperplasia (Hamlin and Banas, 1990). Other explanations for its occurrence postulate altered steroidal homeostasis (Rosol et al., 2013). An exact cause for its exacerbation in mid- and high-dose female rats in the present study is unknown.
Spinal cord degeneration and gliosis and sciatic nerve neuropathy are consistent with previous reports of neurological effects of high doses of acrylamide (Burek et al., 1980, Edwards and Parker, 1977 and O’Shaughnessy and Losos, 1986). The skeletal muscle changes noted in the present study are not clearly associated with sciatic nerve neuropathy. Only fifty percent of the high dose male rats with hind limb skeletal muscle degeneration/necrosis also had sciatic nerve neuropathy. In all other groups there was no association of hind limb myopathy and sciatic neuropathy or between hind limb myopathy and spinal cord degeneration/gliosis in any group. The lack of correlation between spinal cord and sciatic neuropathy with hind limb myopathy is indication that the muscle changes are not neurogenic in origin.
Treatment-related neoplastic changes in acrylamide-treated rats present at the 104-week terminal sacrifice included mammary gland fibroadenomas and thyroid follicular neoplasms. These same tumor target tissues (Table 5) have been reported in prior carcinogenicity studies in Fischer 344 rats (Johnson et al., 1986, Friedman et al., 1995 and Beland et al., 2013). The development of tunica vaginalis mesotheliomas (TVMs) seen in previous acrylamide carcinogenicity studies did not occur in the present study, reflecting the known specific susceptibility of the Fischer 344 rat to Leydig cell tumors and the secondary induction of TVMs (Shipp et al., 2006 and Maronpot et al., 2009).
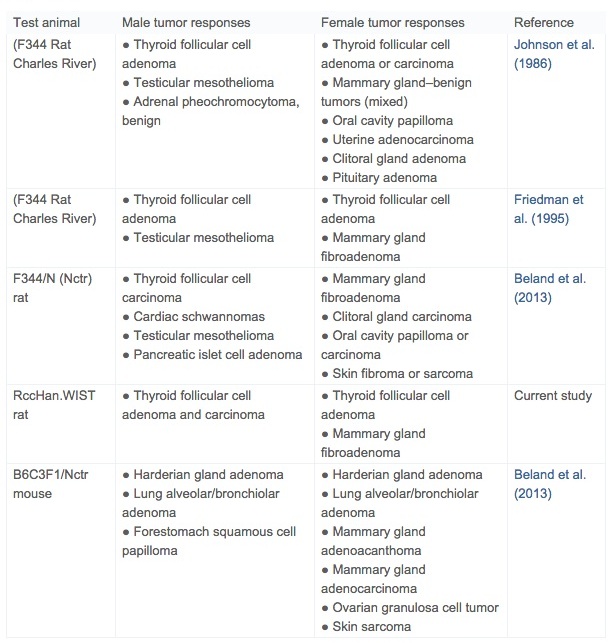
Table 5. Summary of treatment-related tumor responses in conventional two-year carcinogenicity studies of acrylamide.
Mammary gland fibroadenomas have been reported in previous acrylamide rat carcinogenicity studies (Table 5). Rat mammary gland fibroadenomas are typically not considered precursors of malignant mammary adenocarcinomas, although adenocarcinomas rarely arise within fibroadenomas (Rudmann et al., 2012). It is traditionally accepted that the leutotrophic effect of age-associated prolactinemia is causative of rat fibroadenomas and that this mode of action is not relevant to women where prolactin is not leutotrophic (Neumann, 1991 and Ben-Jonathan et al., 2008). Despite some emerging evidence that increased prolactin may be relevant to human breast cancer (Harvey, 2011), in the absence of an increase in malignant mammary gland neoplasia, it is likely that an independent increase in rat mammary fibroadenomas has limited relevance to human cancer risk. The overall incidence of mammary gland fibroadenomas in females in the present study was not statistically significant and is within published control ranges for Wistar rats (Harleman et al., 2012, Tucker, 1997, Hooks et al., 2008 and Poteracki and Walsh, 1998).
Thyroid follicular cell neoplasms were late occurring and without an associated increase in follicular cell hyperplasia. Similar follicular cell neoplastic responses (Table 5) have been documented in previously conducted acrylamide carcinogenicity studies in Fischer 344 rats (Johnson et al., 1986, Friedman et al., 1995 and Beland et al., 2013). Thyroid follicular changes are associated with rat-specific thyroid hormone synthesis, transport and metabolism that differ significantly from thyroid follicular cell hormonal physiology in man (Capen, 1997 and Alison et al., 1994). As opposed to humans, rats are uniquely susceptible to development of thyroid follicular cell tumors because of low affinity thyroxin binding to albumin and 10-fold shorter half-life of T3 and T4 compared to humans (Alison et al., 1994). Consequently, induction of follicular cell neoplasms in chronic rat studies is likely a reflection of a species-specific susceptibility associated with hormonal dysregulation. Interestingly, based on a 14-day study with 2.5 mg/kg/day, hormonal deregulation as a plausible mechanism for thyroid follicular neoplasia in acrylamide treated rats was not supported (Bowyer et al., 2008). However, a role for hormonal dysregulation affecting the pituitary-thyroid axis and the rat-specific thyroid hormonal milieu is generally accepted as an explanation for follicular cell neoplasia in rat carcinogenicity studies (Capen and Martin, 1989, Capen, 1997, Neumann, 1991, Khan et al., 1999 and Alison et al., 1994) and, as such, is a rat-specific response.
There was evidence of thyroid stimulation 7 days after treatment with acrylamide as measured by morphometric decrease in colloid size and increase of follicular cell height (Khan et al., 1999). In Sprague Dawley rats, acrylamide lowers T3 and T4 at 8 weeks after treatment (Hamdy et al., 2012). Acrylamide increased DNA synthesis in thyroids at 7, 14 and 28 days of treatment in both Sprague Dawley rats and Fischer 344 rats as measured by 7 days of bromodeoxyuridine treatment by minipump (Lafferty et al., 2004). However, in a different study following14 days of acrylamide treatment, there was a precipitous drop in Ki-67 protein, a less sensitive marker for proliferation (Bowyer et al., 2008). A modest decrease in T4 was also noted in this study and the authors suggested that an increase in deiodinase expression might account for this effect. An increase in deiodinase would be expected to raise the oxidation levels in thyroid causing oxidative stress (Yousef and El-Demerdash, 2006). Similarly, a correlation of lowered glutathione levels has been correlated with oxidative DNA damage in HEPG2 cells exposed to acrylamide (Jiang et al., 2007). In that study, oxidative damage to DNA was caused by intracellular ROS and glutathione depletion. At pnd 90 and 120 there was a 4 fold increase in reverse T3 suggesting an alternate route of thyroxin metabolism (data not shown).
Another possible explanation for rat thyroid carcinogenicity is a direct action on thyroid DNA. Acrylamide is a weak mutagen, active at high doses (Manjanatha et al., 2006). At doses used in this current study, however, acrylamide mutagenicity is likely below the mutagenic threshold. Mutagenicity has been modeled by Allen using categorical regression (Allen et al., 2005). A BMD10 of 43 mg/kg/day was reported in this study. A threshold of >4 mg/kg/day has been found in the micronucleus assay in rats (Zeiger et al., 2009).
One of the primary purposes for conducting yet another two-year rat carcinogenicity study of acrylamide was to determine if the prior identification of tunica vaginalis mesotheliomas (TVMs) is directly representative of a human health hazard, or, rather, is a consequence of a specific susceptibility of the Fischer 344 rat to development of TVMs. The occurrence of TVMs in Fischer 344 rats is causally linked to their high incidence of Leydig cell tumors and altered steroidogenesis (Alison et al., 1994, Shipp et al., 2006 and Maronpot et al., 2009). In the present study in Wistar Han male rats, there were no TVMs diagnosed and only one Leydig cell tumor was seen in a mid-dose rat (Table 4). Consequently, a plausible mode of action for the TVMs seen in the two previous acrylamide two-year rat studies is related to dysregulation of the testicular hormonal milieu and the physical effect of the high incidence of Leydig cell tumors—both effects specific to Fischer 344 rats. Evidence for dysregulation of the testicular hormonal milieu has been previously reported (Shipp et al., 2006 and Hamdy et al., 2012). Other possible treatment-related neoplasia identified in previous rat carcinogenicity studies including marginal increases in clitoral gland carcinomas, cardiac schwannomas, islet cell adenomas, and oral cavity papillomas and carcinomas did not occur in the present study. The occurrence of these marginal tumor responses in different tissues carry similar concerns regarding relevance as TVMs and their assessment in terms of human health risk is best dealt with in a weight-of-evidence approach to risk assessment.
In the present study the observed tumor responses were limited to the thyroid and mammary glands. Both of these target tissue tumor responses have rat-specific modes of action suggesting less likelihood of human cancer risk than previously estimated.
In conclusion, a two-year carcinogenicity study conducted in Wistar Han rats beginning with in utero exposure had excellent survival with clear indication that the 3.0 mg/kg/day high dose was consistent with a maximum tolerated dose. The study resulted in a statistically significant increased incidence of thyroid follicular neoplasms in males and females and in a non-statistically significant increase in mammary fibroadenomas in females. In addition, evidence of axonal degeneration and hind limb weakness were present in high dose males and females. The absence of TVMs in the present study indicates that their previous documentation in rat carcinogenicity studies is a Fischer 344 male rat specific response. In addition, epidemiologic studies of dietary acrylamide intake have failed to demonstrate an increased risk of cancer for human (Lipworth et al., 2012).
Acknowledgments
This work was supported by SNF SAS, ZAC de Milieux, Andrézieux, France. The authors thank Dr. Marvin A. Friedman for his advice and insight.
References
Alison et al., 1994
R.H. Alison, C.C. Capen, D.E. Prentice
Neoplastic lesions of questionable significance to humans
Toxicol Pathol, 22 (1994), pp. 179–186
Allen et al., 2005
B. Allen, E. Zeiger, G. Lawrence, M. Friedman, A. Shipp
Dose-response modeling of in vivo genotoxicity data for use in risk assessment: some approaches illustrated by an analysis of acrylamide
Regul Toxicol Pharmacol, 41 (2005), pp. 6–27
Beland et al., 2013
F.A. Beland, P.W. Mellick, G.R. Olson, M.C. Mendoza, M.M. Marques, D.R. Doerge
Carcinogenicity of acrylamide in B6C3F (1) mice and F344/N rats from a 2-year drinking water exposure
Food Chem Toxicol, 51 (2013), pp. 149–159
Ben-Jonathan et al., 2008
N. Ben-Jonathan, C.R. LaPensee, E.W. LaPensee
What can we learn from rodents about prolactin in humans
Endocr Rev, 29 (2008), pp. 1–41
Bowyer et al., 2008
J.F. Bowyer, J.R. Latendresse, R.R. Delongchamp, L. Muskhelishvili, A.R. Warbritton, M. Thomas, et al.
The effects of subchronic acrylamide exposure on gene expression, neurochemistry, hormones, and histopathology in the hypothalamus-pituitary-thyroid axis of male Fischer 344 rats
Toxicol Appl Pharmacol, 230 (2008), pp. 208–215
Burek et al., 1980
J.D. Burek, R.R. Albee, J.E. Beyer, T.J. Bell, R.M. Carreon, D.C. Morden, et al.
Subchronic toxicity of acrylamide administered to rats in the drinking water followed by up to 144 days of recovery
J Environ Pathol Toxicol, 4 (1980), pp. 157–182
Capen and Martin, 1989
C.C. Capen, S.L. Martin
Mechanisms that lead to disease of the endocrine system in animals
Toxicol Pathol, 17 (1989), pp. 234–249
Capen, 1997
C.C. Capen
Mechanistic data and risk assessment of selected toxic end points of the thyroid gland
Toxicol Pathol, 25 (1997), p. 39
Damjanov and Friedman, 1998
I. Damjanov, M.A. Friedman
Mesotheliomas of tunica vaginalis testis of Fischer 344 (F344) rats treated with acrylamide: a light and electron microscopy study
In vivo, 12 (1998), pp. 495–502
Edwards and Parker, 1977
P.M. Edwards, V.H. Parker
A simple, sensitive, and objective method for early assessment of acrylamide neuropathy in rats
Toxicol Appl Pharmacol, 40 (1977), pp. 589–591
EPA, 2010
EPA
Toxicological review of acrylamide
U.S. Environmental Protection Agency, report EPA/635/R-07/009FEPA, Washington, DC (2010)
EPA, 2008
EPA Science Advisory Board
Toxicological review of acrylamide
U.S. Environmental Protection Agency, report EPA/635/R-07/009FEPA Science Advisory Board, Washington, DC (2008)
Erdreich and Friedman, 2004
L.S. Erdreich, M.A. Friedman
Epidemiologic evidence for assessing the carcinogenicity of acrylamide
Regul Toxicol Pharmacol, 39 (2004), pp. 150–157
Friedman, 2003
M. Friedman
Chemistry, biochemistry, and safety of acrylamide. A review
J Agric Food Chem, 51 (2003), pp. 4504–4526
Friedman, 2005
M. Friedman
Biological effects of Maillard browning products that may affect acrylamide safety in food: biological effects of Maillard products
Adv Exp Med Biol, 561 (2005), pp. 135–156
Friedman et al., 1995
M.A. Friedman, L.H. Dulak, M.A. Stedham
A lifetime oncogenicity study in rats with acrylamide
Fundam Appl Toxicol, 27 (1995), pp. 95–105
Greaves, 2012
P. Greaves
Histopathology of preclinical toxicity studies interpretation and relevance in drug safety evaluation
Academic Press, Amsterdam (2012)
Hamdy et al., 2012
S.M. Hamdy, H.M. Bakeer, E.F. Eskander, O.N. Sayed
Effect of acrylamide on some hormones and endocrine tissues in male rats
Hum Exp Toxicol, 31 (2012), pp. 483–491
Hamlin and Banas, 1990
M. Hamlin, D. Banas
Adrenal gland
G.A. Boorman, M. Eustis, C. Montgomery Jr., W. MacKenzie (Eds.), Pathology of the Fischer rat, Academic Press, San Diego (1990), pp. 501–518
Harleman et al., 2012
J.H. Harleman, A. Hargreaves, H. Andersson, S. Kirk
A review of the incidence and coincidence of uterine and mammary tumors in Wistar and Sprague-Dawley rats based on the RITA database and the role of prolactin
Toxicol Pathol, 40 (2012), pp. 926–930
Harvey, 2011
P.W. Harvey
Prolactin-induced mammary tumorigenesis is not a rodent-specific response
Toxicol Pathol, 39 (2011), pp. 1020–1022
Hooks et al., 2008
W. Hooks, C. Groom, M-C. Laurent, I. Taylor
Tumour data from Han Wistar rat dietary and oral gavage tumorigenicity studies, completed over the period of 1994–2006
Toxicol Lett, 180 (2008), pp. S68–S69
JECFA, 2005
JECFA
Joint FAO/WHO Expert Committee on food additives
WHO/FAO, Rome (2005), p. 47
Jiang et al., 2007
L. Jiang, J. Cao, Y. An, C. Geng, S. Qu, L. Jiang, et al.
Genotoxicity of acrylamide in human hepatoma G2 (HepG2) cells
Toxicol In Vitro, 21 (2007), pp. 1486–1492
Johnson et al., 1986
K.A. Johnson, S.J. Gorzinski, K.M. Bodner, R.A. Campell, C.H. Wolf, M.A. Friedman, et al.
Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats
Toxicol Appl Pharmacol, 85 (1986), pp. 154–168
Khan et al., 1999
M.A. Khan, C.A. Davis, G.L. Foley, M.A. Friedman, L.G. Hansen
Changes in thyroid gland morphology after acute acrylamide exposure
Toxicol Sci, 47 (1999), pp. 151–157
Laast et al., 2014
V.A. Laast, T. Larsen, N. Allison, M.J. Hoenerhoff, G.A. Boorman
Distinguishing cystic degeneration from other aging lesions in the adrenal cortex of Sprague-Dawley rats
Toxicol Pathol, 42 (2014), pp. 823–829
Lafferty et al., 2004
J.S. Lafferty, L.M. Kamendulis, J. Kaster, J. Jiang, J.E. Klaunig
Subchronic acrylamide treatment induces a tissue-specific increase in DNA synthesis in the rat
Toxicol Lett, 154 (2004), pp. 95–103
Lipworth et al., 2012
L. Lipworth, J.S. Sonderman, R.E. Tarone, J.K. McLaughlin
Review of epidemiologic studies of dietary acrylamide intake and the risk of cancer
Eur J Cancer Prev, 21 (2012), pp. 375–386
Manjanatha et al., 2006
M.G. Manjanatha, A. Aidoo, S.D. Shelton, M.E. Bishop, L.P. McDaniel, L.E. Lyn-Cook, et al.
Genotoxicity of acrylamide and its metabolite glycidamide administered in drinking water to male and female Big Blue mice
Environ Mol Mutagen, 47 (2006), pp. 6–17
Maronpot et al., 2009
R.R. Maronpot, E. Zeiger, E.E. McConnell, H. Kolenda-Roberts, H. Wall, M.A. Friedman
Induction of tunica vaginalis mesotheliomas in rats by xenobiotics
Crit Rev Toxicol, 39 (2009), pp. 512–537
Mottram et al., 2002
D.S. Mottram, B.L. Wedzicha, A.T. Dodson
Acrylamide is formed in the Maillard reaction
Nature, 419 (2002), pp. 448–449
Neumann, 1991
F. Neumann
Early indicators for carcinogenesis in sex-hormone-sensitive organs
Mutat Res, 248 (1991), pp. 341–356
O’Shaughnessy and Losos, 1986
D.J. O’Shaughnessy, G.J. Losos
Comparison of central and peripheral nervous system lesions caused by high-dose short-term and low-dose subchronic acrylamide treatment in rats
Toxicol Pathol, 14 (1986), pp. 389–394
Poteracki and Walsh, 1998
J. Poteracki, K.M. Walsh
Spontaneous neoplasms in control Wistar rats: a comparison of reviews
Toxicol Sci, 45 (1998), pp. 1–8
Rosol et al., 2013
T. Rosol, R. DeLellis, P. Harvey, C. Sutcliffe
Endocrine system
W. Haschek, C. Rousseaux, M. Wallig, B. Bolon, R. Ochoa, B. Mahler (Eds.), Haschek and Rousseaux’s handbook of toxicologic pathology, Elsevier, Amsterdam (2013), pp. 2392–2492
Rudmann et al., 2012
D. Rudmann, R. Cardiff, L. Chouinard, D. Goodman, K. Kuttler, H. Marxfeld, et al.
Proliferative and nonproliferative lesions of the rat and mouse mammary, Zymbal’s, preputial, and clitoral glands
Toxicol Pathol, 40 (Suppl) (2012), pp. 7S–39S
Shipp et al., 2006
A. Shipp, G. Lawrence, R. Gentry, T. McDonald, H. Bartow, J. Bounds, et al.
Acrylamide review of toxicity data and dose–response analyses for cancer and noncancer effects
Crit Rev Toxicol, 36 (2006), pp. 481–608
Tucker, 1997
M.J. Tucker
Diseases of the Wistar rat
Taylor & Francis, London; Bristol, PA (1997)
Vesper et al., 2010
H.W. Vesper, S.P. Caudill, J.D. Osterloh, T. Meyers, D. Scott, G.L. Meyers
Exposure of the U.S. population to acrylamide in the National Health and Nutrition Examination Survey 2003–2004
Environ Health Perspect, 118 (2010), pp. 278–283
Vesper et al., 2008
H.W. Vesper, N. Slimani, G. Hallmans, A. Tjonneland, A. Agudo, V. Benetou, et al.
Cross-sectional study on acrylamide hemoglobin adducts in subpopulations from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study
J Agric Food Chem, 56 (2008), pp. 6046–6053
Wall, 2005
H. Wall
Acrylamide a two-year drinking water chronic toxicity study in Fischer 344 rats
A histopathology review and pathology working group review of proliferative lesions involving the mesothelial lining cells of the tunica vaginalis of the testes in a two-year drinking water chronic toxicity study in Fischer 344 rats with acrylamideExperimental Pathology Laboratories, Inc. (2005) (750-001)
Yousef and El-Demerdash, 2006
M.I. Yousef, F.M. El-Demerdash
Acrylamide-induced oxidative stress and biochemical perturbations in rats
Toxicology, 219 (2006), pp. 133–141
Zeiger et al., 2009
E. Zeiger, L. Recio, T.R. Fennell, J.K. Haseman, R.W. Snyder, M. Friedman
Investigation of the low-dose response in the in vivo induction of micronuclei and adducts by acrylamide
Toxicol Sci, 107 (2009), pp. 247–257