Mexico City (MC) residents are exposed to severe air pollution and exhibit olfactory bulb inflammation. We compared the olfactory function of individuals living under conditions of extreme air pollution to that of controls from a relatively clean environment and explore associations between olfaction scores, apolipoprotein E (APOE) status, and pollution exposure. The olfactory bulbs (OBs) of 35 MC and 9 controls 20.8+/-8.5 years were assessed by light and electron microscopy. The University of Pennsylvania Smell Identification Test (UPSIT) was administered to 62 MC/25 controls 21.2+/-2.7 years. MC subjects had significantly lower UPSIT scores: 34.24+/-0.42 versus controls 35.76+/-0.40, p=0.03. Olfaction deficits were present in 35.5% MC and 12% of controls. MC APOE epsilon 4 carriers failed 2.4+/-0.54 items in the 10-item smell identification scale from the UPSIT related to Alzheimer’s disease, while APOE 2/3 and 3/3 subjects failed 1.36+/-0.16 items, p=0.01. MC residents exhibited OB endothelial hyperplasia, neuronal accumulation of particles (2/35), and immunoreactivity to beta amyloid betaA(42) (29/35) and/or alpha-synuclein (4/35) in neurons, glial cells and/or blood vessels. Ultrafine particles were present in OBs endothelial cytoplasm and basement membranes. Control OBs were unremarkable. Air pollution exposure is associated with olfactory dysfunction and OB pathology, APOE 4 may confer greater susceptibility to such abnormalities, and ultrafine particles could play a key role in the OB pathology. This study contributes to our understanding of the influences of air pollution on olfaction and its potential contribution to neurodegeneration.
Keywords: α synuclein, Alzheimer’s disease, amyloid β 42, air pollution, APOE, olfaction, olfactory bulb, Parkinson’s disease, ultrafine particulate matter
Introduction
Air pollution is a complex mixture of particulate matter (PM), gases, and organic compounds present in outdoor and indoor air. Children living in Mexico City (MC) exhibit evidence of chronic inflammation of the upper and lower respiratory tracts, accumulation of particulates in nasal respiratory epithelium, breakdown of the nasal respiratory epithelial barrier, systemic inflammation, brain inflammation, cognitive deficits, and MRI brain abnormalities (Calderon-Garciduenas et al., 2001a, 2003a, 2007a, b, 2008a–c). Ultrafine particulate matter (UFPMo100 nm) is found in the olfactory bulbs (OBs) of children and young adults exposed to the highly polluted atmosphere of MC (Calderon-Garciduenas et al., 2007a, 2008b). Healthy dogs living in MC also exhibit disruption of nasal and olfactory barriers, increased apurinic and apyrimidinic DNA sites in olfactory bulb and hippocampus tissues, and white matter hyperintense prefrontal lesions by MRI similar to those present in children (Calderon-Garciduenas et al., 2001b, 2003b, 2008c).
Adult residents in MC exhibit up-regulation of a powerful inflammatory gene, cyclooxygenase-2 (COX2), in the olfactory bulb and other brain regions (Calderón-Garcidueñas et al., 2004). Children and adults younger than 25 years exhibit up-regulation of COX2, interleukin 1 beta (IL1β), and the key innate immunity receptor CD14 in their olfactory bulbs (Calderón-Garcidueñas et al., 2008b). Immunoreactivity (IR) to Aβ42 is present in mitral and tufted olfactory neurons as well as olfactory ensheathing cells and astrocytes, while α synuclein IR in the form of Lewy neurites and cytoplasmic deposits is also seen in olfactory bulb neurons of young MC residents (Calderón-Garcidueñas et al., 2008b). The observation that MC teenagers with an APOE ε 4 allele accumulate Aβ42 in olfactory bulb neurons concomitantly with markers of oxidative stress, mitochondrial abnormalities, and dysfunction of the proteasomal system (Jung et al., 2007, Keller 2006) is very important. The APOE ε 4 allele is a major genetic risk factor for the development of Alzheimer’s disease and older adult ε 4 carriers perform poorly on odor identification tests (Corder et al., 1993, Graves et al., 1999, Kovacs et al., 2004,Calhoun et al., 2005, Handley et al., 2006; Olofsson et al., 2008). Also relevant to our study, Kozauer et al., has shown an association between cognitive decline and APOE ε 4 in young individuals. Specifically, ε 4 carriers first seen at an average age of 29.3 years and followed-up for 22 years scored lower on the Mini-Mental State Examination (MMSE) and three tests of verbal learning: immediate recall, delayed recall and word recognition (Kozauer et al., 2008). Kozauers’ study suggests that the association between APOE ε 4 and cognitive decline is likely an early one, emphasizing the need to explore olfactory deficits in younger individuals carrying the ε 4 allele.
The goals of this study were threefold: first, to compare the olfactory function of a cohort of healthy young adults residing in MC to that of a matched low pollution cohort; second, to assess if the carriers of an ε 4 APOE allele would have significantly more olfactory deficits compared to APOE 2/3 and 3/3 carriers in both the high and the low pollution cohorts; third, to characterize the olfactory bulb pathology in a matched cohort of low and high pollution exposed residents. We selected as our olfactory test the Spanish version of the University of Pennsylvania Smell Identification Test (UPSIT) (Doty et al., 1984; Doty, 1995). Ten items within this test have been shown to strongly predict conversion from mild cognitive impairment to Alzheimer’s disease (Tabert et al., 2005). Given that olfactory bulb pathology has been observed in MC residents exposed to high levels of pollution, this study also examined the pathology of the olfactory bulbs of 35 MC and 9 matched controls. We hypothesized that: i. odor identification scores would be lower in MC subjects compared to controls; ii. carriers of the APOE 4 allele residing in MC would perform more poorly on the 10 UPSIT items related to risk for Alzheimer’s disease; and iii. olfactory bulb pathology would be significant in the MC residents.
Methods
Study Areas
Mexico City represents an extreme of urban growth and environmental pollution (Bravo-Alvarez and Torres-Jardón, 2002; Molina et al., 2007). The Mexico City Metropolitan Area (19°25′N latitude and 99° 100′ W longitude) lies in an elevated basin at an altitude of 2240 meters above mean sea level and its urbanized area covers around 2000 km2. The basin is surrounded by high mountains ridges on the east, south, and west but with a broad opening to the north and a gap to the south-southwest. The surrounding mountains combined with the frequent morning thermal inversions contribute to trap and accumulate air pollutants inside the basin. In this geographical setting, 20 million residents, nearly 4 million vehicles, and over 40 000 industries consume more than 40 million liters of petroleum fuels per day emitting significant concentrations of primary air pollutants (Molina et al., 2007). The high altitude and tropical climate of the region is highly conductive to fast photochemistry forming secondary pollutants such as ozone (O3) and particulate matter (PM).
The northwest sector of MC -the residency area of our exposed cohort- corresponds to a mixed medium income residential and industrial area with heavy traffic. Although PM10 (particulate matter with aerodynamic diameters of less than 10 μm) and PM2.5 (particulate matter with aerodynamic diameters of less than 2.5 μm) concentrations over this sector are not the highest in the metropolitan area, their levels represent a health concern to its residents (Secretaría del Medio Ambiente del Gobierno del Distrito Federal, 2006). The typical coarse fraction (PM2.5-10) in Mexico City is ∼ 54% while the fine fraction (PM<2.5) is ∼ 46%, the latest fraction relates to traffic exhaust emissions (Vega et al., 2004;Querol et al., 2008). The control subjects lived in Polotitlán (20°13′N latitude and 99° 49′W longitude), a rural town of ∼ 3,000 inhabitants located 121 km NW of MC at 2380 m above mean sea level. Its main activity is agricultural with just a few small textile manufacturing and dairy products facilities. The prevalent wind direction at Polotitlán comes from rural not polluted areas and thus insures good air quality. The PM10 levels at Polotitlán are ∼ 5 % lower than that of the NWMC and it is estimated that the coarse PM fraction is ∼ 60% (due to the influence of local soil resuspension), while the fine fraction is ∼ 40% (Secretaría de Ecología del Gobierno del Estado de México, 2005, Querol et al., 2008). Data from the control town indicated that other criteria air pollutants (ozone, sulfur dioxide, carbon monoxide and nitrogen dioxides) were below the current EPA standards (Secretaría de Ecología del Gobierno del Estado de México, 2005). The selection of this rural control town was based on 4 key additional factors: i. access to a young adult healthy population, ii. previous clinical studies with the Polotitlán cohort that indicated that children had no evidence of air pollution-related health issues (Calderón-Garcidueñas et al., 2007b, 2008b), iii. an altitude above sea level similar to that of MC, and iv. its relative proximity to MC to facilitate clinical access of the cohorts.
Clinical study population
The MC cohort included 62 subjects, 21 women and 41 males, with a mean (SD) age of 21.1 (2.6) years. The mean number of years of education was 13.6 (0.7). Their average time spent outdoors was 4.43 (0.5) hours per day. The control subjects included 14 males and 11 females
Olfactory Testing Protocol
The study was approved by the Universidad del Ejército Human Studies Committees, and written consent was obtained from all subjects. Olfactory function was quantified using the Spanish version of the University of Pennsylvania Smell Identification Test (UPSIT). This self-administered standardized test incorporates 40 microencapsulated odorants and a forced-choice multiple alternative format to establish both absolute (i.e., normosmia, anosmia, or mild, moderate or severe hyposmia) and relative (percentile ranks) indices of function (Doty, 1995). We analyzed the full 40 item score as well as the 10 Item score that strongly predicts conversion to Alzheimer’s disease (AD) on follow-up evaluation in patients with mild cognitive impairment (Tabert et al. 2005). All data were managed anonymously. Subjects were studied in December 2005 and June 2008.
Pathology Study
Autopsies and tissue preparation
The mean (SD) age of the 35 MC autopsy subjects was 19.2 (6.7) years (range 2 – 32 years), and for the 9 controls residents 21.3 (10.3) years (range 2– 40 years). The MC cohort included 16 children ranging in age from 2 to 17 years [mean (SD) = 13.5 (4.7) years] and the control cohort included a 2 y old girl and three 17y old boys [mean (SD) = 13.2 (7.5)]. Subjects were elementary, middle, high school, and college students and blue and white collar workers. Three APOE 4 carriers were identified in the MC group and none in the control group. All autopsied subjects were clinically healthy and had died suddenly.
All 44 subjects had full autopsies, including complete neuropathological examinations and were included in the immunohistochemistry (IHC) studies. The selected cases had no pathological evidence of disease processes other than the acute cause of death. Autopsies were performed 4.1 ± 1.1 h after death. The skull was opened and the olfactory bulbs and the brain removed. Brain sections were immersed in 10% neutral formaldehyde, fixed for 48 h, and transferred to 70% alcohol. Olfactory bulb and nerve sections were included for this study. Paraffin sections 5-7 μm thick were cut and routinely stained with hematoxylin and eosin, and used for immunohistochemistry. Immunohistochemistry (IHC) was performed on olfactory bulbs sections. The sections were deparaffinized, and immunostained as described previously (Calderón-Garcidueñas et al., 2004, 2008a). In this work we used 88% formic acid as an epitope retrival method for the Aβ, while for α synuclein we used a proteinase K protocol 10 (Beach et al., 2008). Negative controls included omission or substitution of primary antibodies by nonspecific, isotype-matched antibodies. Positive (Alzheimer’s patients) and negative controls were included for each antibody. Confirmation of the IR was done with different antibodies in serial sections with a minimum of 10 slides for each Ab in each case (mean 14±2.2 SD). Selected antibodies included: β amyloid 1-16 (6E10 Signet), Covance, Emeryville, CA 1:2000, β amyloid, 17-24 (4G8 Signet), Covance, Emeryville, CA, 1:1000, α-synuclein LB509 (InVitrogen, Carlsbad, CA 1:800), α-synuclein ab 2080 to aa 116-131 and ab24592 to residues Y 125 and Y 136 (Abcam Cambridge, Mass 1:1000), PHF-Tau-8 (Innogenetics, Belgium, AT-8, 1:100), and glial fibrillary acidic protein GFAP (Abcam, Cambridge, Mass 1:500). Sections were reviewed by three pathologists with no access to the codes regarding the identification data. Electron microscopy was performed in 10 olfactory bulb samples (5 controls, 5 MC) fixed in 2% paraformaldehyde and 2% glutaraldehyde in sodium phosphate buffer (0.1M, pH 7.4), post-fixed in 1% osmium tetraoxide and embedded in Epon. Semithin sections (0.5 – 1 μm) were cut, stained with toluidine blue for light microscopy examination. Ultrathin sections (60- 90 nm) were cut and collected on slot grids previously covered with formvar membrane. Sections were stained with uranyl acetate and lead citrate and examined with a Carl Zeiss EM109T (Germany) or a JEM-1011 (Japan) microscope.
Apolipoprotein E genotyping
APOE genotype information was obtained through analysis of either nasal or venous samples for the clinical participants and from brain samples in the autopsy cases. Samples were genotyped using Taqman ready to use assays from both SNP’s that constitute the APOE genotype according to TaqMan Gene Expression Assays, Applied Biosystems, 2006.
Data analysis
In the olfactory study, the primary variables of interest were the total UPSIT scores, the subset of UPSIT items known to be particularly sensitive to Alzheimer’s disease, residency time in MC, and the APOE alleles. The two-sample Wilcoxon rank sum (Mann-Whitney) test was used for comparison for variables of interest between Apo E 2/3 and 3/3 and 3/4 and 4/4 subjects. All statistical computations were performed with the use of Stata 8.3 software (Stata Corp, College Station, TX). A two-sided type I error rate of 0.05 was considered statistically significant.
Results
Olfactory Function
The mean UPSIT scores for MC residents were lower than that of their matched controls [means (SEMs) 34.24 (0.42) and 35.76 (0.40); p=0.03, with the average deficit reflecting mild microsmia. Table 1 summarizes the olfactory scores, including those for the different APOE alleles in the two cohorts. Olfaction deficits ranging from mild to severe microsmia- regardless of APOE status- were identified in 35.5% of the MC residents, while 12% of the control residents had only mild microsmia. There were no significant differences in the total UPSIT scores between the MC APOE ε 4 and the APO E 2/3 or 3/3 genotype carriers [mean (SD) = 33.3 (4.2) and 34.46 (3.0); p = 0.52]. That being said, MC residents having the APOE 4 allele failed significantly more items from the 10 UPSIT items known to be particularly sensitive to AD than their APOE 2/3 or 3/3 allele MC counterparts [respective mean (SEM) scores = 2.4 (0.54) & 1.3 (0.16), p = 0.01], a finding not observed in the control subjects (p = 0.08). This is in spite of the fact that the APOE 2 and 3 subjects lived, on average, four more years in MC than did the APOE ε 4 subjects [respective mean years (SD) = 6.54 (1.09) & 2.4 (0.6); p=0.02]. No significant UPSIT score differences were observed between Control APOE ε 4 carriers and Control APO E 3/3 carriers [respective means (SD) = 35.0 (1.1) & 35.9 (2.1); p = 0.31].
Human Pathology
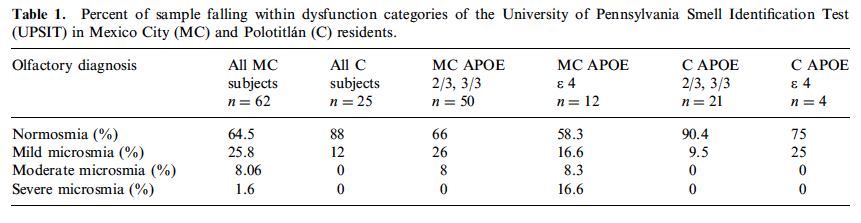
Gross brain examination was unremarkable in all subjects. The control olfactory bulbs were negative for Aβ42, α-synuclein and PHF-Tau-8 and were unremarkable on microscopic examination. However, this was not the case for the olfactory bulbs of the MC cohort. Two of the 16 Mexico City children-boys age 14 and 17 y- exhibited significant amounts of black particulate material in the cytoplasm of neuron specific enolase (NSE) + cells around glomeruli (Figure 1 A). Amyloid Aβ42 (with both β 6E10 and 4G8 Signet Ab), was seen in ensheathing cells, as well as in astrocytes in the olfactory nerve and olfactory bulb neurons including the anterior olfactory nucleus, in 29 of 35 subjects (Figure 1 B), the youngest being a 2-year-old-boy with an APOE 3/3 genotype. Amyloid Aβ42 was also present in the smooth muscle cells of arteriolar subarachnoid blood vessels and in endothelial cells in small capillaries of 2/29 MC subjects. Isolated Aβ42 diffuse plaques were seen in 5 MC subjects (ages: 13, 15, 18, 22 & 26 years). Alpha synuclein was present in the form of granular punctuate cytoplasmic deposits in the glomerular, mitral and granular cell layers in 4/35 of subjects (Figure 1C), and 2 had also Lewy neurites. Two subjects had both Aβ42 and α-synuclein, while 2 subjects had only α-synuclein. In only six subjects (all APOE 3/3) we could not identified the IR for Aβ42 or α-synuclein (age 18.17±1.47). All 3 APOE 4 carriers exhibited Aβ42 and the only homozygous was also α-synuclein positive. None of the subjects in this study had PHF-Tau-8 IR. Corporae amylacea were numerous along the length of the olfactory nerves starting in the late 10′s; the numbers were significantly increased in subjects with an APOE 4. Significant amounts of yellow-brown material consistent with lipofuscin were seen both in endothelial cells and olfactory bulb neurons in teens (Figure 1D and insert). Thick arteriolar vessel walls were observed with significant enlargement of the Virchow-Robin spaces (Figure 1E). Endothelial hyperplasia was significant in arterioles and capillaries and a few vessels exhibited a marked reduction in their lumen and polymorphonuclear leukocytes attached to their walls (Figure 1F). Electron micrographs of arterioles in the olfactory bulb revealed ultrafine particulate matter in the range of 16-28 nm in the cytoplasm of endothelial cells, and endothelial basement membranes (Figure 2A). The basic laminar olfactory bulb organization: glomerular, external plexiform layer, mitral cell layer, internal plexiform layer and granule cell layer was preserved in control subjects (Figure 2B), however exposed matched subjects displayed ill-defined and fragmented organization with very small, loose glomeruli (Figure 2C). APOE 4 young carriers exhibited the most laminar disorganization and the smallest, ill defined glomeruli.
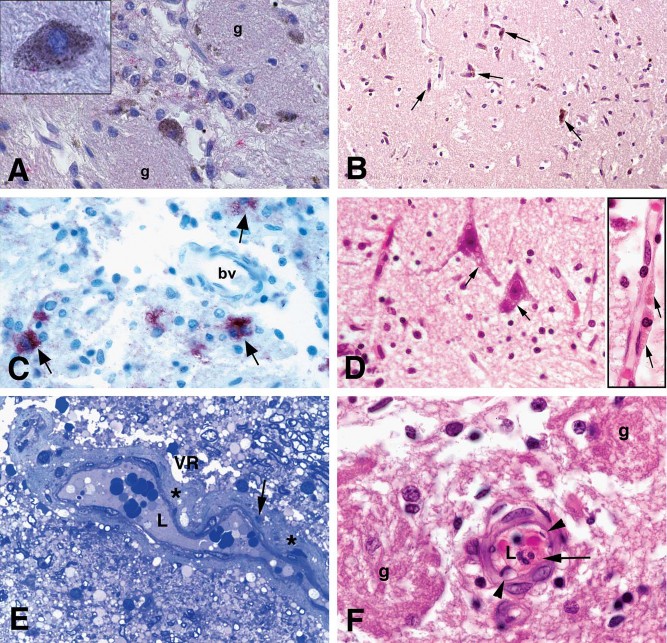
Fig 1. A. Olfactory bulb neurons in the glomerular region (g) exhibit abundant cytoplasmic particulate matter in a 14 year old MC boy. Upper left insert: a close-up of one PM-loaded neuron with positive Aβ 42 red product in its cytoplasm. Aβ 42 IHC counterstained with hematoxylin B. Olfactory bulb in a 13y old girl showing granular positive staining for Aβ42 in anterior olfactory nucleus neurons (arrows, brown product). C. Olfactory bulb in an 11 year old MC boy APOE ε 3/3 showing granular positive staining for α synuclein in olfactory neurons (arrows red product). α synuclein IHC D. The mitral layer in a 14y old male APOE ε 3/3. Mitral neurons (arrows) exhibit abundant lipofuscin, also present abundantly in endothelial cells (insert, arrows). H&E E. Olfactory bulb arteriole in a 14 y MC girl. Notice the thickening of the vessel wall (*), and the presence of cell debris within the wall (arrow). There is a significant enlargement of the Virchow-Robin space (VR). The lumen in blood vessel is marked L. One micron toluidine blue section. F. Olfactory bulb blood vessel in a 14y boy. Notice a polymorphonuclear leucocyte (PMN long arrow) attached to the vessel wall, and the vacuolated endothelial cells (arrow heads). Glomeruli are marked g. H&E
Fig 2. A. Electron micrograph of a small olfactory bulb arteriole in a 17 y male. Two sharply defined particles are seen in the endothelial cytoplasm (EC) and the basement membrane (BM) of the endothelial cell (arrows). The particles in the nano size range measure 16 and 20 nm. The smooth muscle cell is identified as SMC and the vessel lumen as L. EM × 50,000
B. Olfactory bulb in a 20y old control male. The laminar organization is intact, bundles of the olfactory nerve (ofn) are seen approaching the mitral cell layer (arrows). The granule cell layer (gcl) is composed of multiple small round neurons with layering organization. The external plexiform layer is present (epl). H&E
C. Eleven year MC boy with few mitral neurons (arrows), small loose glomeruli (g) and blood vessels with thick cellular walls and reduced lumen (arrow head). The granule cell layer (gcl) and the external plexiform layer are identified (epl). H&E
Air Quality Data
Air quality MC data clearly indicate that, during the period of the study, residents in NW Mexico City were exposed to significant concentrations of PM10 and PM2.5. The annual PM2.5 average concentrations from 2004 to June 2008 registered by the local official monitoring network (Partisol PM2.5 samplers) in MC, ranged from 23.6 to 24.3 μg/m3 (Secretaría del Medio Ambiente del Gobierno del Distrito Federal, 2008). The interpolated annual PM2.5 average concentration for the study area (parabolic interpolation method as suggested in EPA, 1977) for any 12-month period combination between December 2005 and June 2008 was 21.03 μg/m3 (9.28 μg/m3 SD). For comparison, the annual mean air quality standard for PM2.5 stands at 15.0 μg/m3. In terms of short-exposure 24-hr levels, the exploratory statistical analysis for the NWMC December 2005 – June 2008 data period indicated that in 8 % of this period (∼ 77 days) PM2.5 24-hour concentrations were above 35 μg/m3, while the median was 19.9 μg/m3 and the maximum was 57. 35 μg/m3. In general, PM2.5 composition or its spatial distribution in MC has not changed significantly in the last 10 years (Vega et al., 2004; Querol et al., 2008). Organic carbon (OC) has been shown to be the major component, accounting for ∼ 48% (Stone et al., 2008). Secondary inorganic aerosols are the second major component of PM2.5, accounting for 26%, and elemental carbon (soot) resulting from incomplete combustion accounts for ∼ 17%.
Discussion
Environmental risk factors have been implicated in the development of neurodegenerative diseases such as Alzheimer’s and Parkinson’s, and a disturbed sense of smell is seen early in the course of these two major neurodegenerative diseases (Doty, 1991,1993,2008; Hawkes 2003; Kovacs 2004; Strous and Shoenfeld 2006; Berendse and Ponsen, 2006). Occupational exposures to airborne particulates and aerosolized metals (i.e., welding), have been associated with smell loss and some forms of central nervous system degeneration (Tjalve and Henriksson 1999; Antunes et al., 2007). The present study suggests that a relationship exists between olfactory deficits in young healthy individuals and their sustained exposures to a complex mixture of air pollutants. Thus, more than a third of Mexico City young healthy adults had odor identification deficits independently of APOE status. Moreover, carriers of an APOE ε 4 allele with less residency time in MC failed significantly more UPSIT items known to be sensitive to Alzheimer’s disease than their APOE 2/3 and 3/3 MC and control counterparts, implying that a combination of environmental factors and genetics plays a role in influencing olfactory function.
The pathology findings in Mexico City children and young adults indicate that particulate matter accumulates in the respiratory nasal epithelium, olfactory epithelium and Bowman glands, as well as in olfactory bulb (OB) neurons (Calderón-Garcidueñas et al., 2001a, 2008b). The presence of UFPM in the endothelium and basement membranes of olfactory bulb arterioles was associated with endothelial inflammation, as shown by neutrophils attaching to the vessel walls, and endothelial hyperplasia with significant reduction of vessel lumen (Calderón-Garcidueñas et al., 2008b; Pober et al. 2008). MC subjects exhibited olfactory bulb/nerve immunoreactivity of βA42 in 83% and α-synuclein in 11% of cases, indicating that the abnormal protein accumulation is a very common response in highly exposed subjects.
At least five critical olfactory bulb-related issues could be pertinent to subjects exposed to air pollution: i. the olfactory transport into the brain of toxic materials including particulate matter (Tjalve and Henriksson 1999; Mascagni et al., 2003; Dorman et al., 2006, Chen et al., 2008). ii. the role of ultrafine particulates (UFPM) in the enhancement rate of protein fibrillation potentially affecting amyloid β42and alpha synuclein (Linse et al., 2007; Colvin and Kulinowski 2007; Cedervall et al., 2007a,b; Lynch et al., 2007), iii. the OB accumulation of beta-amyloid1-42 and alpha-synuclein (Selkoe 2002; Jellinger 2003; Hawkes 2003), iv. the OB neuroinflammation observed in individuals exposed to air pollutants (Calderón-Garcidueñas et al., 2004, 2008b), and v. the impact of the OB pathology upon the neuronal populations, including the migrating progenitor cells from the subventricular zone (SVZ) (Doetsch et al., 1999; Bedard and Parent 2004; Alvarez-Buylla and Lim 2004; Lledo et al., 2008; Petzold et al., 2008).
The olfactory nerve/OB pathway is a well known route of access of toxins and PM to the brain for experimental animals and occupational exposures (Tjalve and Henriksson 1999; Mascagni et al., 2003;Elder et al., 2006). Both the olfactory and the trigeminal (responsible for the nasal perception of cold, pungent or burning sensations) pathways are likely portals of entry in urban residents (Calderón-Garcidueñas et al., 2008b). There has been a growing interest on the identification of fine and ultrafine PM in urban air, and their health effects (Donaldson 2003; Fang et al., 2005; Peters et al., 2006). Ultrafine particles have a very large surface-to-volume ratio, and are not membrane bound which allows for direct access to intracellular proteins, organelles and DNA, enhancing their toxic potential (Geiser et al., 2005; Klein 2007). The release of nanoparticles to the environment as aerosols from traffic, waste and industry processes strongly suggest that inhalation is an important access route in humans and dogs (Hagens et al., 2007). Transport of nanoparticles across an epithelium are dependant on concentration, temperature and size (des Rieux et al., 2005). Chen et al., have recently published a paper showing the effects of aluminium oxide nanoparticles in brain endothelial cell cultures and intact rats. Nano-alumina produced significant endothelial oxidative stress and disrupted the expression of tight junction proteins (Chen et al., 2008). These experimental observations are very relevant to MC subjects, since we have described alterations of zonula occludens (ZO-1) immunoreactivity with a breakdown of the BBB in the frontal cortex of exposed children (Calderón-Garcidueñas et al., 2008b). Factors such as age, gender, weight, race, nostril shape, exercise level, minute ventilation, and outdoor time all contribute to the particle deposition, and to lesser or higher risk from inhalation of pollutant PM in ambient air (Bennett and Zeman 2005). Approximately 5-20% of the nasal air flow passes through the olfactory region (Hahn et al., 1993), and in a recent human nasal computational fluid dynamic model using particles ranging in size from 5-50 μm and volumetric flow rates of 7.5, 15 and 30 L/min, the olfactory region had a PM deposition efficiency maximum value of 3% (Schroeter et al., 2006). These data are critical for children given that their respiratory frequency is higher than adults and thus their PM olfactory deposition could be higher.
The novel observation that nanoparticles can significantly enhance the rate of protein fibrillation (Colvin and Kulinowski 2007; Lynch et al., 2007; Linse et al., 2007; Klein 2007; Cedervall et al., 2007 a, b) and the ability of synthetic polymers to interact and alter polypeptide conformations (Heegaard et al., 2005) adds a new important facet to the issue of environmental (natural and man-made) nanoparticles playing a role in neurodegeneration.
The mammalian olfactory bulb receives new neurons throughout life, progenitors migrate from the subventricular zone (SVZ) located in the walls of the lateral ventricles facing the stratium, the septum and the corpus callosum (Altman 1969; Kaplan and Hinds, 1977; Bédard and Parent 2004; Lledo et al., 2008). Within the olfactory bulb, young neurons mature into various types of local inhibitory interneurons (Lledo et al., 2008). Different interneuron subtypes are produced at different ages and play a crucial role in olfactory processing, thus neuronal accumulation of ultrafine particles and/or abnormal proteins could likely alter the plasticity of postnatal olfactory circuits and impair olfactory circuits with functional consequences. Furthermore, the endothelial and basement membrane changes we are describing in olfactory bulb vessels likely also alter the delicate balance between the neuro-glial-vascular components of the olfactory glomeruli (Petzold et al., 2008; Shepherd and Charpak 2008). Specifically, in these structures there is a close interaction between axon terminals, presynaptic and postsynaptic dendrites, glial cells and the capillary/arteriolar network. Thus, gliosis, alterations in the Virchow-Robin spaces, breakdown of the BBB, endothelial hyperplasia, reduction of the arteriolar lumen and thickening of arteriolar walls could alter the blood perfusion in relation to neuronal activity in an anatomical and functional unit onto which all olfactory sensory axons that express the same odor receptors converge (Mombaerts et al., 1996; Petzold et al., 2008).
Evidence that olfactory bulb pathology is clearly associated with olfactory dysfunction is illustrated very well in both Alzheimer and Parkinson’s diseases (AD and PD) (Ansari and Johnson 1975; Doty 2003, Kovacs 2004). Olfactory loss is a very early finding in both AD and PD, and precedes cognitive and motor symptoms respectively by years (Hawkes 2003; Kranick and Duda 2008). Odor identification deficits in APOE ε 4 allele AD siblings (ages 59-88 y) are considered to be early cognitive markers of incipient AD (Handley et al., 2006). In older adults, unexplained olfactory dysfunction in the presence of an ε 4 allele is associated with a high risk of cognitive decline (OR 4.9) (Graves et al., 1999). APO ε 4 allele subjects have a more rapid decline in odor identification than in odor threshold or dementia rating scale scores (Calhoun-Haney et al., 2005). Thus, our findings of APOE ε 4 subjects failing more items in the 10-item smell identification scale related to AD raises a key question: does residency in a highly polluted city accelerate olfactory deficits associated with increased risk for AD? In this small study, APOE 4 subjects had significantly less residency time in MC (p=0.02) compared to their APOE 2 and 3 counterparts, but displayed the same deficits in UPSIT scores, along with significantly increased failure rates in the 10 UPSIT items most sensitive to AD. This suggests there may be an acceleration of their olfaction deficits. Given that functional brain abnormalities and cognitive decline are detected in young APOE 4 carriers decades before the clinical onset of dementia, it remains to be determined whether olfactory deficits precede the abnormally low rates of cerebral glucose metabolism in the posterior cingulate, parietal, temporal and prefrontal cortex and/or the lowered Mini-Mental and verbal learning test scores (Reiman et al., 2004; Kozauer et al., 2008).
Our findings suggest olfactory tests may be of value along with cognitive testing in young persons with complaints of olfactory dysfunction but with no known risk factors for dementia (Handley et al., 2006). Physicians should be aware that exposures to polluted urban environments can result in olfactory deficits. In the United States alone, 29 million people are exposed to PM10, 88 million to PM2.5, and millions more to PM occupationally and in the setting of disasters, including war, fires, and the aftermath of terrorist attacks such as occurred at the World Trade Center (Desai et al., 2009).
The long term significance of olfactory deficits in young individuals residing in a highly polluted environment, particularly those carrying an APOE 4 allele, remains to be defined. However, we are of the opinion that such individuals living in regions of high pollution are likely of high risk for later development of progressive olfactory and cognitive deficits, including AD and PD. This possibility is based upon our findings of pollution-related (a) neuroinflammation in key brain areas, (b) altered innate immune brain responses, (c) presence of PM in olfactory neurons, endothelial cells and their basement membranes, and (d) the accumulation of beta amyloid 42 and alpha-synuclein in olfactory bulb, supra and infratentorial locations (Calderón-Garcidueñas et al., 2004, 2008 b,c).
Acknowledgments
This work supported in part by the National Science Foundation 0346458, the Montana Board of Research and Commercialization Technology 04-06 to Rafael Villarreal-Calderon, NCRR Grant #P20 RR015583, and P30 ES013508. This work was presented in part at the Neurotoxicology 25th Meeting, October 13, 2008, Rochester, NY, USA, by Rodolfo Villarreal-Calderon. RL Doty is President and major shareholder in Sensonics, Inc., the manufacturer of the olfactory tests used in this study.
References
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457.
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain.Neuron. 2004;41:683–686.
- Ansari KA, Johnson A. Olfactory dysfunction in patients with Parkinson’s disease. J Chronic Dis.1975;28:493–497.
- Antunes M, Bowler RM, Doty RL. San Francisco/Oakland Bay Bridge Welder Study: olfactory function. Neurology. 2007;69:1278–1284.
- Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW, Leverenz JB, Roncaroli F, Buttini M, Hladik CL, Sue LI, Nooringian JV, Adler CH. Evaluation of α-synuclein immunohistochemical methods used by invited speakers. Acta Neuropath. 2008;116:277–288.
- Bédard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Brain Res Dev Brain Res. 2004;151:159–168.
- Berendse HW, Ponsen MM. Detection of preclinical Parkinson’s disease along the olfactory tract.J Neural Transm Suppl. 2006;70:321–325.
- Bennett WD, Zeman KL. Effect of race on fine particle deposition for oral and nasal breathing.Inhal Toxicol. 2005;17:641–648.
- Bravo-Alvarez HR, Torres-Jardón R. Air pollution levels and trends in the México City metropolitan area. In: Fenn M, de Bauer L, Hernández T, editors. Urban Air Pollution and Forest: Resources at Risk in the Mexico City Air Basin Ecological Studies. Vol. 156. Springer-Verlag; New York: 2002. pp. 121–159.
- Calderón-Garcidueñas L, Valencia-Salazar G, Rodríguez-Alcaraz A, Gambling TM, Osnaya N, Villarreal-Calderón A, Devlin RB, Carson JL. Ultrastructural nasal pathology in children chronically and sequentially exposed to air pollutants. Am Respir Crit Care Med. 2001a;24:132–138.
- Calderón-Garcidueñas L, Mora-Tiscareño A, Fordham LA, Chung CJ, Osnaya N, Hernández J, Acuña H, Gambling TM, Villarreal-Calderón A, Carson J, Koren H. S, Devlin RB. Canines as sentinel species for assessing chronic exposures to air pollutants: Part 1. Respiratory Pathology.Toxicol Sci. 2001b;61:342–355.
- Calderón-Garcidueñas L, Mora-Tiscareño A, Fordham LA, Valencia-Salazar G, Chung CJ, Rodriguez-Alcaraz A, Paredes R, Variakojis D, Villarreal-Calderon A, Flores-Camacho L, Antunez-Solis A, Henriquez-Roldan C, Hazucha MJ. Respiratory damage in children exposed to urban pollution. Pediatric Pulmonology. 2003a;36:148–61.
- Calderón-Garcidueñas L, Maronpot RR, Torres-Jardon R, Henríquez-Roldán C, Schoonhoven R, Acuna-Ayala H, Villarreal-Calderón A, Nakamura J, Fernando R, Reed W, Azzarelli B, Swenberg JA. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol.2003b;31:524–538.
- Calderón-Garcidueñas L, Reed W, Maronpot RR, Henríquez-Roldán C, Delgado-Chavez R, Calderón-Garcidueñas A, Dragustinovis I, Franco-Lira M, Aragón-Flores M, Solt AC, Altenburg M, Torres-Jardón R, Swenberg JA. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol. 2004;32:650–658.
- Calderón-Garcidueñas L, Franco-Lira M, Torres-Jardón R, Henriquez-Roldán C, Valencia-Salazar G, Gonzalez-Maciel A, Reynoso-Robles R, Villarreal-Calderon R, Reed W. Pediatric respiratory and systemic effects of chronic air pollution exposure: nose, lung, heart and brain pathology. Toxicol Pathol. 2007a;35:154–162.
- Calderón-Garcidueñas L, Vincent R, Mora-Tiscareño A, Franco-Lira M, Henríquez-Roldán C, Garrido-Garcia L, Camacho-Reyes L, Valencia-Salazar G, Paredes R, Romero L, Osnaya H, Villarreal-Calderon R, Torres-Jardon R, Hazucha MJ, Reed W. Elevated plasma endothelin-1 and pulmonary arterial pressure in children exposed to air pollution. Environ Health Perspect.2007b;115:1248–53.
- Calderón-Garcidueñas L, Villarreal-Calderon R, Valencia-Salazar G, Henríquez-Roldán C, Gutiérrez-Castrellón P, Torres-Jardon R, Osnaya-Brizuela N, Romero L, Coria R, Solt A, Reed W. Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhal Toxicol. 2008a;20:499–506.
- Calderón-Garcidueñas L, Solt A, Franco-Lira M, Henríquez-Roldán C, Torres-Jardón R, Nuse B, Herritt L, Villarreal-Calderon R, Osnaya N, Brooks DM, Gonzalez-Maciel A, Reynoso-Robles R, Delgado-Chavez R, Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain-barrier, ultrafine particle deposition, and accumulation of amyloid beta 42 and alpha synuclein in children and young adults. Toxicol Pathol. 2008b;36:289–310.
- Calderón-Garcidueñas L, Mora-Tiscareño A, Gómez-Garza G, Broadway J, Chapman S, Valencia-Salazar G, Jewells V, Maronpot RR, Henriquez-Roldan C, Pérez-Guillé B, Torres-Jardon R, Herritt L, Brooks D, Osnaya-Brizuela N, Monroy ME, Gonzalez-Maciel A, Reynoso-Robles R, Villarreal-Calderon R, Solt AC, Engle RW. Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain and Cognition. 2008b;68:117–127.
- Calhoun-Haney R, Murphy C. Apolipoprotein ε 4is associated with more rapid decline in odor identification than in odor threshold or Dementia rating Scale scores. Brain and Cognition.2005;58:178–182.
- Cedervall T, Lynch I, Foy M, Berggård T, Donnelly SC, Cagney G, Linse S, Dawson KA. Detailed identification of plasma proteins adsorbed on Copolymer nanoparticles. Angew Chem Int Ed. 2007a;46:5754–5756.
- Cedervall T, Lynch I, Lindman S, Berggård T, Thulin E, Nilsson H, Dawson KA, Linse S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA. 2007b;104:2050–2055.
- Chen L, Yokel RA, Henning B, Toborek M. Manufactured aluminum oxide nanoparticles decrease expression of tight junction proteins in brain vasculature. J Neuroimmune Pharmacol.2008;3:286–295.
- Colvin VL, Kulinowski KM. Nanoparticles as catalysts for protein fibrillation. PNAS.2007;104:8679–8680.
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923.
- Des Rieux A, Ragnarsson EGE, Gullberg E, Préat V, Schneider YJ, Artursson P. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. Eur J Pharmac Sci. 2005;25:455–465.
- Desai SC, Doty RL, de la Hoz R, Moline J, Herbert R, Gannon PJ, Altman KW. Prevalence and severity of smell dysfunction in workers at the post-9/11 World Trade Center site. American Journal of Rhinology. 2009 submitted.
- Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural ítem cells in the adult mammalian brain. Cell. 1999;97:703–716.
- Donaldson K. The biological effects of coarse and fine particulate matter. Occup Environ Med.2003;60:313–314.
- Dorman DC, Struve MF, Marshall MW, Parkinson CU, James RA, Wong BA. Tissue manganese concentrations in young male rhesus monkeys following subchronic manganese sulphate inhalation. Toxicol Sci. 2006;92:201–210.
- Doty RL. Development of the University of Pennsylvania Smell Identification Test: A standardized microencapsulated test of olfactory function. Physiol Behav (Monograph)1884;32:489–502.
- Doty RL, Perl DP, Steele JC, Chen KM, Pierce JD, Reyes P, Kurland LT. Olfactory dysfunction in three neurodegenerative diseases. Geriatrics. 1991;46(Suppl 1):47–51.
- Doty RL. The Smell Identification Test™ Administration Manual. 3rd. Haddon Hts., NJ: Sensonics, Inc.; 1995.
- Doty RL. Odor perception in neurodegenerative diseases and schizophrenia. In: Doty RL, editor.Handbook of Olfaction and Gustation. 2nd. NY: Marcel Dekker; 2003. pp. 479–502.
- Doty RL. The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol.2008;63:7–15.
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, Potter R, Maynard A, Ito Y, Finkelstein J, Oberdörster G. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Persp. 2006;114:1172–1178.
- EPA. EPA-450/2-77-024a. U.S. Environmental Protection Agency; Research Triangle Park, North Carolina 27711: 1977. Guideline on procedures for constructing air pollution isopleth profiles and population exposure analysis.
- Fang GC, Wu YS, Wen CC, Lin CK, Huang SH, Rau JY, Lin CP. Concentrations of nano and related ambient air pollutants at the traffic sampling site. Toxicol Ind Health. 2005;21:259–271.
- Geiser M, Rothen-Rutishauser B, Kapp N, Schürch S, Kreyling W, Schulz H, Semmler M, Im Hof V, Heyder J, Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113:1555–1560.
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon status. Neurology. 1999;53:1480–1487.
- Hagens WI, Oomen AG, de Jong WH, Cassee FR, Sips AJAM. What do we (need to) know about the kinetic properties of nanoparticles in the body? Reg Toxicol Pharmacol. 2007;49:217–229.
- Handley OJ, Morrison CM, Miles C, Bayer AJ. ApoE gene and familial risk of Alzheimer’s disease as predictors of odour identification in older adults. Neurobiol Aging. 2006;27:1425–1430.
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094.
- Hahn I, Scherer PW, Mozell MM. Velocity profiles measured for airflow through a large-scale model of the human nasal cavity. J Appl Physiol. 1993;75:2273–2287.
- Hawkes C. Olfaction in neurodegenerative disorders. Mov Disord. 2003;18:364–372.
- Heegaard PMH, Boas U, Otzen DE. Dendrimer effects on peptide and protein fibrillation.Macromol Biosci. 2007;7:1047–1059.
- Jellinger KA. Neuropathological spectrum of Synucleopathies. Movement Disorders.2003;18:S2–S12.
- Jung T, Bader N, Grune T. Lipofuscin. Ann NY Acad Sci. 2007;1119:97–111.
- Keller JN. Age-related neuropathology, cognitive decline, and Alzheimer’s disease. Ageing Res Rev. 2006;5:1–13.
- Klein J. Probing the interactions of proteins and nanoparticles. Proc Natl Acad Sci USA.2007;104:2029–2030.
- Kozauer AN, Mielke MM, Chan GKC, Rebock GW, Lyketsos CG. Apolipoprotein E genotype and lifetime cognitive decline. Int Psychogeriatrics. 2008;20:109–123.
- Kovács T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders.Ageing Res Rev. 2004;3:215–232.
- Kranick SM, Duda JE. Olfactory dysfunction in Parkinson’s disease. Neurosignals. 2008;16:35–40.
- Linse S, Cabaleiro-Lago C, Lynch I, Lindman S, Thulin E, Radford SE, Dawson KA. Nucleation of protein fibrillation by nanoparticles. Proc Natl Acad Sci USA. 2007;104:8691–8696.
- Lledo PM, Merkle FT, Alvarez-Buylla A. Origen and function of olfactory bulb interneuron diversity. Cell. 2008;31:392–400.
- Lynch I, Cedervall T, Lundqvist M, Cabaleiro-Lago C, Linse S, Dawson KA. The nanoparticle-protein complex as a biological entity: a complex fluids and surface science challenge for the 21st century. Adv Colloid Interface Sci. 2007:134–135. 167–174.
- Mascagni P, Consonni D, Bregante G, Chiappino G, Toffoletto F. Olfactory function in workers exposed to moderate airborne cadmium levels. Neurotoxicol. 2003;24:717–724.
- Molina LT, Kolb CE, de Foy B, Lamb BK, Brune WH, Jimenez JL, Ramos-Villegas R, Sarmiento J, Paramo-Figueroa VH, Cárdenas B, Gutierrez-Avedoy V, Molina MJ. Air quality in North America’s most populous city – overview of the MCMA-2003 campaign. Atmos Chem Phys. 2007;7:2447–2473.
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686.
- Olofsson JK, Nordin S, Wiens S, Hedner M, Nilsson LG, Larson M. Odor identification impairment in carriers of ApoE-ε4 is independent of clinical dementia. Neurobiol Aging. 2008 in press.
- Peters A, Veronesi B, Calderón-Garcidueñas L, Gehr P, Chen LC, Geiser M, Reed W, Rothen-Rutishauser B, Schürch S, Schulz H. Translocation and potential neurological effects of fine and ultrafine particles: a critical update. Part Fiber Tox. 2006;3:1–13.
- Petzold GC, Albeanu DF, Sato TF, Murthy VN. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 2008;58:897–910.
- Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury and death. Ann Rev Pathol. 2008 Aug 28; E Pub.
- Querol X, Pey J, Minguillón MC, Pérez N, Alastuey A, Viana M, Moreno T, Bernabé RM, Blanco S, Cárdenas B, Vega E, Sosa G, Escalona S, Ruiz H, Artíñano S. PM speciation and sources in Mexico during the MILAGRO-2006 Campaign. Atmos Chem Phys. 2008;8:111–128.
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. PNAS. 2004;101:284–289.
- Secretaría del Medio Ambiente del Gobierno del Distrito Federal. Dirección General de Gestión Ambiental del Aire, Sistema de Monitoreo Atmosférico. Ciudad de México: 2006. La Calidad del Aire en la Zona Metropolitana del Valle de México, 1986-2005.
- Secretaría del Medio Ambiente del Gobierno del Distrito Federal. SIMAT – Sistema de Monitoreo Atmosférico de la Ciudad de México. Nov, 2008. online available at:http://www.sma.df.gob.mx/simat/, data downloaded in.
- Secretaría de Ecología del Gobierno del Estado de México. Dirección General de Prevención y Control de la Contaminación Atmosférica. Departamento de Monitoreo Ambiental; Tlalnepantla de Baz, Estado de México: 2005. Medición de la Calidad del Aire y Parámetros Meteorológicos en el Municipio de Polotitlán. Noviembre-Diciembre 2004.
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791.
- Schroeter JD, Kimbell JS, Asgharian B. Analysis of particle deposition in the turbinate and olfactory regions using a human nasal computational fluid dynamics model. J Aerosol Med.2006;19:301–313.
- Shepherd GM, Charpak S. The olfactory glomerulus: a model for neuro-glio-vascular biology.Neuron. 2008;58:827–829.
- Stephens S, Madronich S, Wu F, Olson J, Ramos R, Retama A, Muñoz S. Weekly patterns of México City’s surface concentrations of CO, NOx, PM10, and O3 during 1986-2007. Atmos Chem Phys Discuss. 2008;8:8357–8384.
- Stone EA, Snyder DC, Sheesley RJ, Sullivan AP, Weber RJ, Schauer JJ. Source apportionment of fine organic aerosol in Mexico City during the MILAGRO experiment 2006. Atmos Chem Phys. 2008;8:1249–1259.
- Strous RD, Shoenfeld Y. To smell the immune system: Olfaction, autoimmunity and brain involvement. Autoimmunity Reviews. 2006;6:54–60.
- Tabert MH, Liu X, Doty RL, Serby M, Zamora D, Pelton GH, Marder K, Albers MW, Stern Y, Devanand DP. A 10-Item smell identification scale related to risk for Alzheimer’s disease.Annals Neurol. 2005;58:155–160.
- Tjalve H, Henriksson J. Uptake of metals in the brain via olfactory pathways. Neurotoxicology.1999;20:181–195.
- Vega E, Reyes E, Ruiz H, García J, Sánchez G, Martínez-Villa G, González U, Chow JC, Watson JG. Analysis of PM2.5 and PM10 in the Atmosphere of Mexico City during 2000–2002. J Air & Waste Manag Assoc. 2002;54:786–798.

