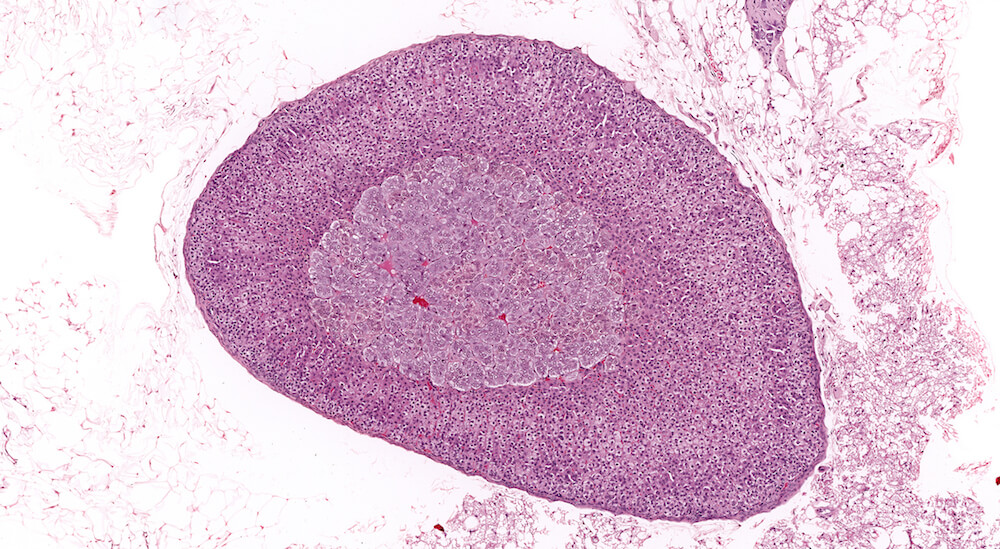
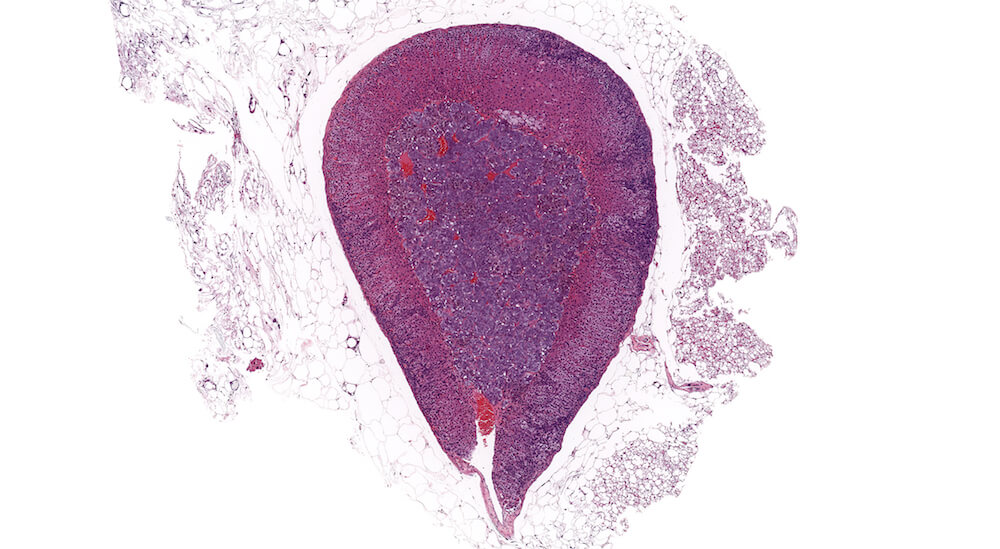
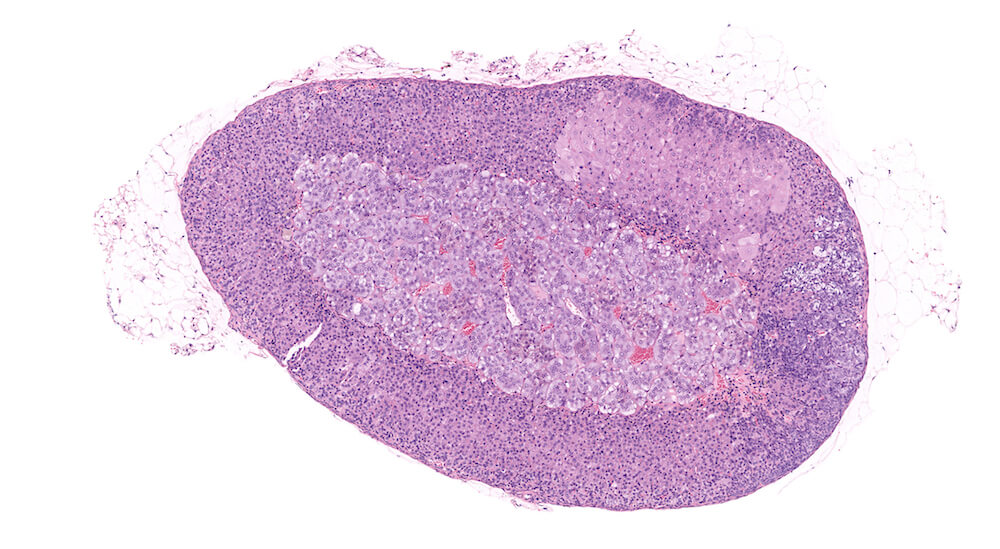
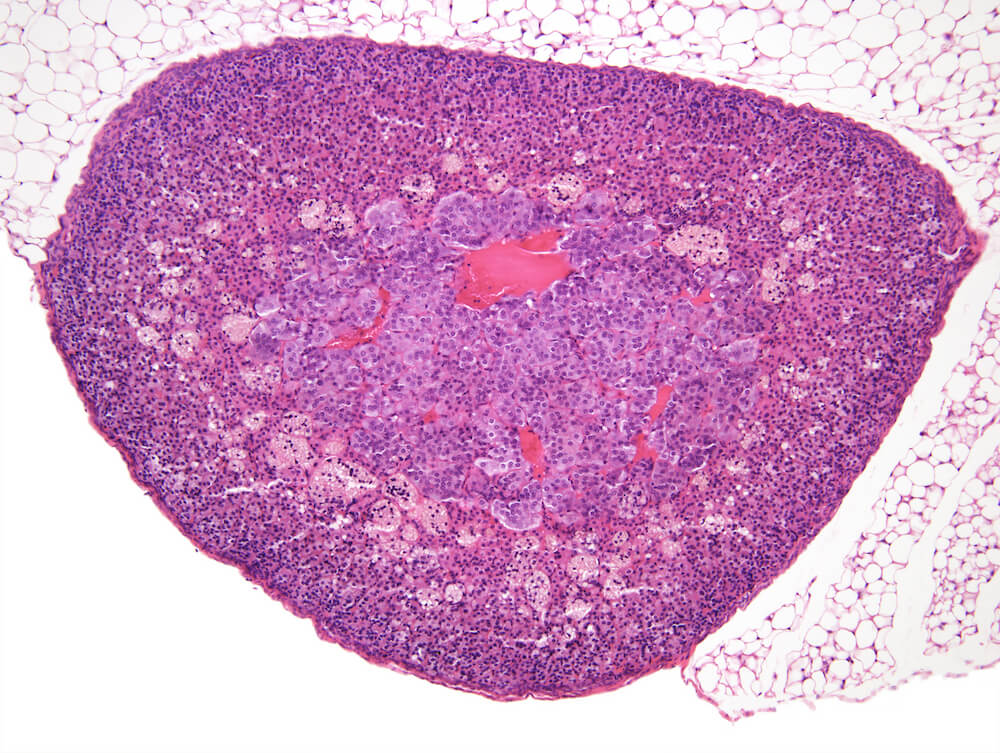
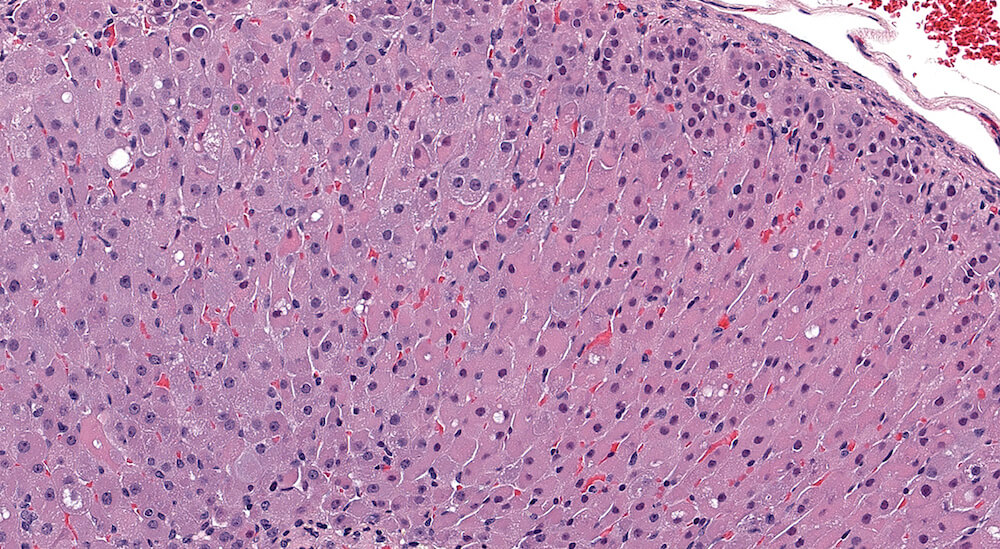
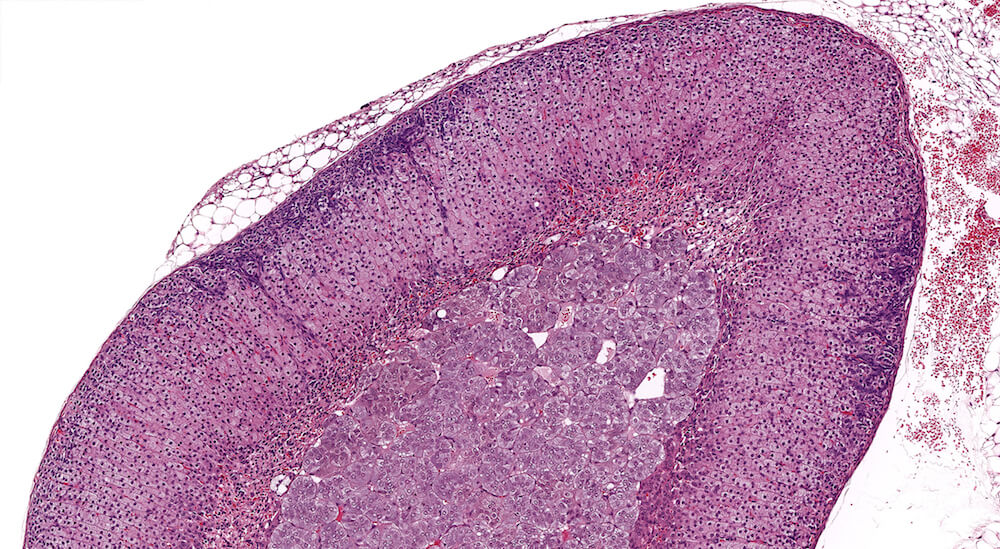
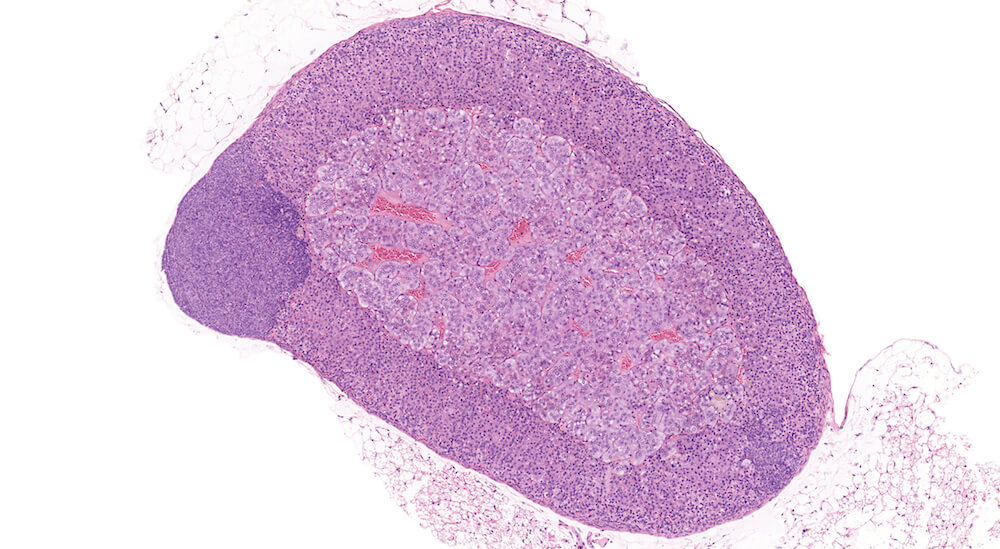
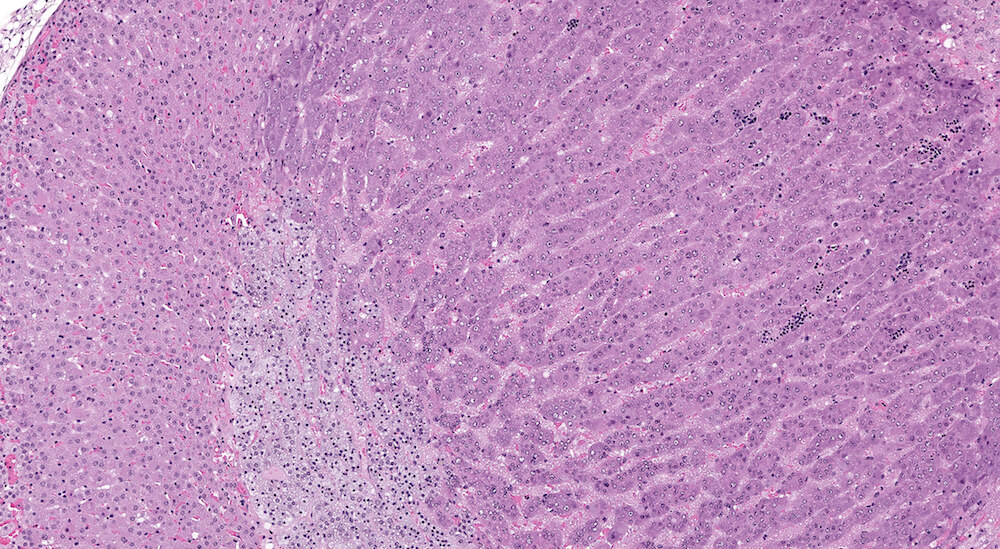
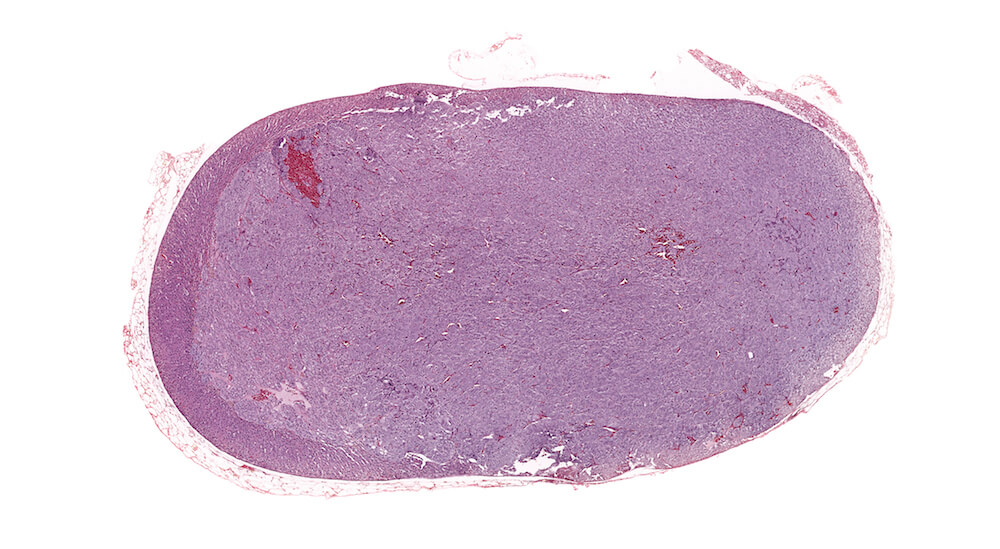
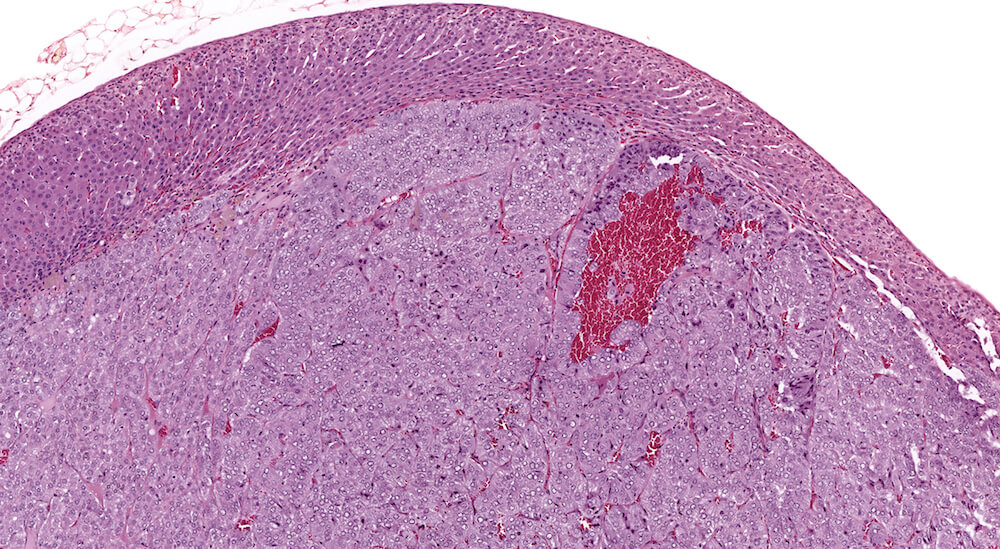
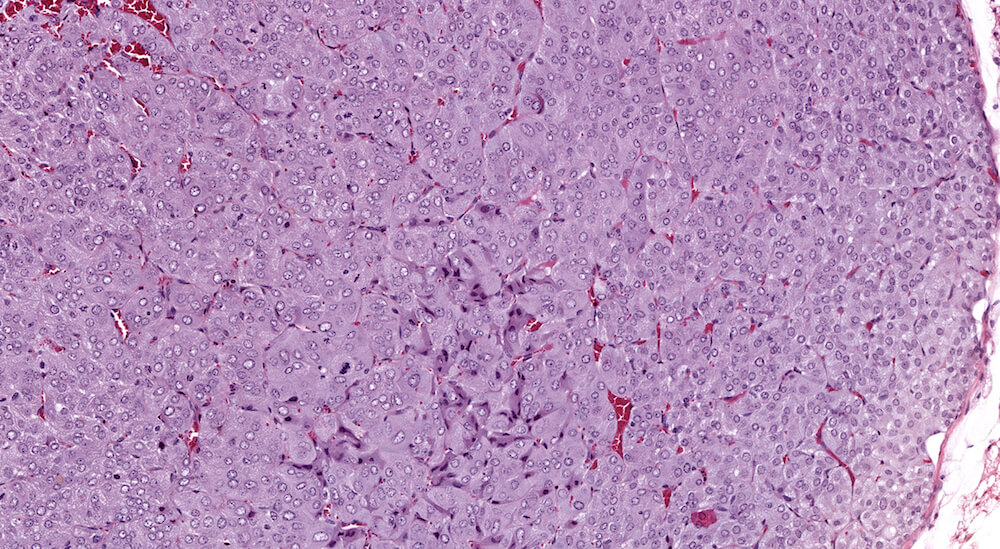
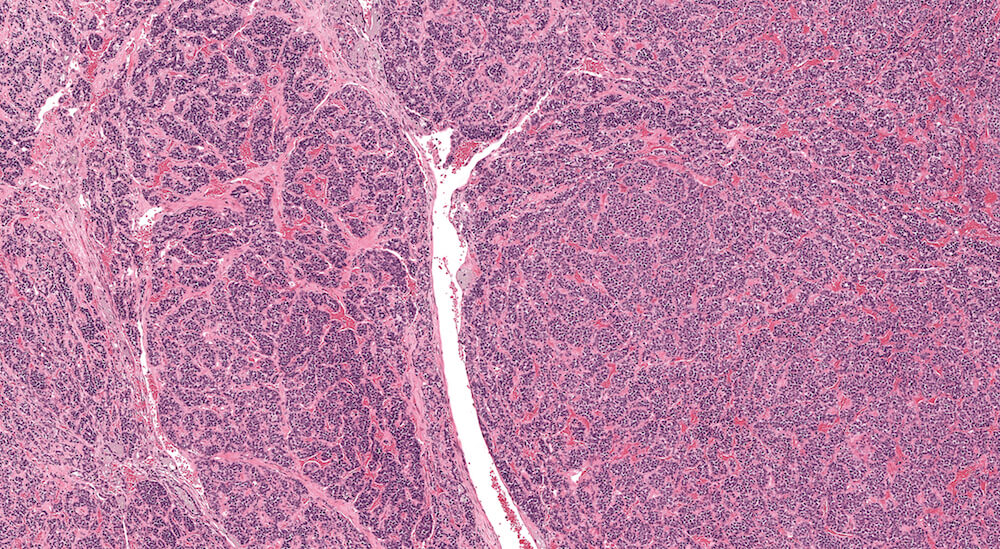
Figure 1. Normal, Gestation Day 18. B6C3F1 mouse. The structure of the adrenal gland a gestation day 18 clearly shows the zona glomerulosa, zona fasciculata, and the medulla. Zona glomerulosa cells are arranged in small nests or packets and Zona fasciculata cells have begun to align in columns perpendicular to the capsular surface. Some intermingling of zona fasciculata and medulla cells can be seen as the corticomedullary junction is not sharply delineated.
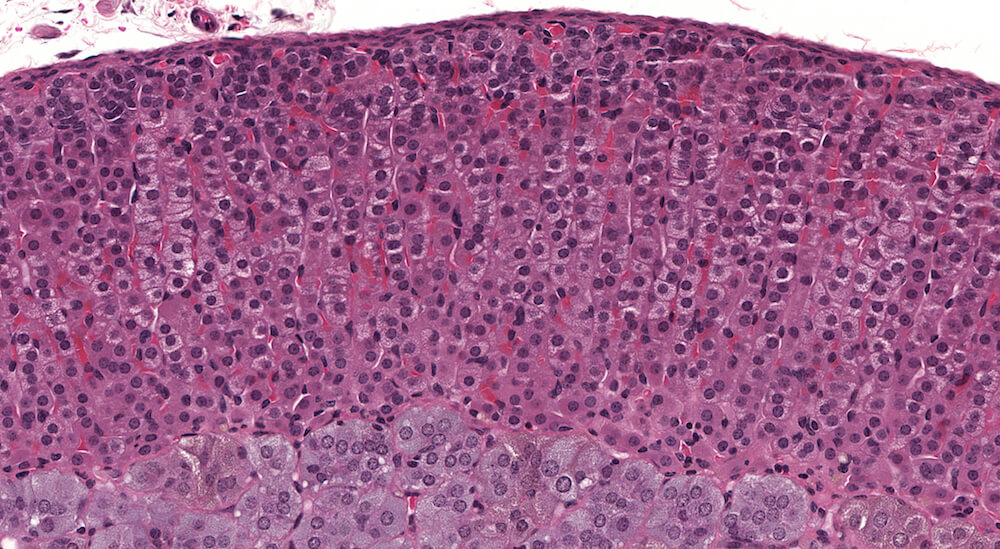
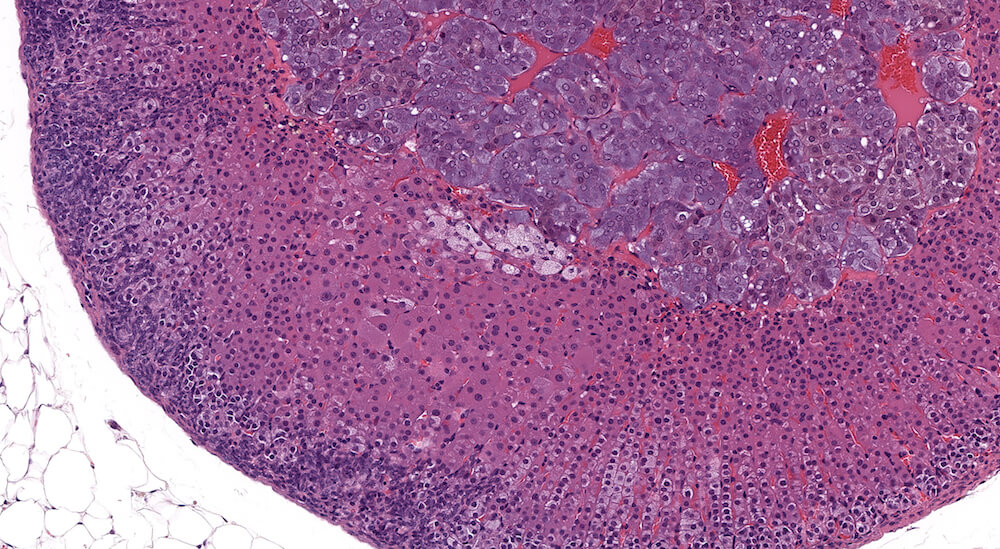
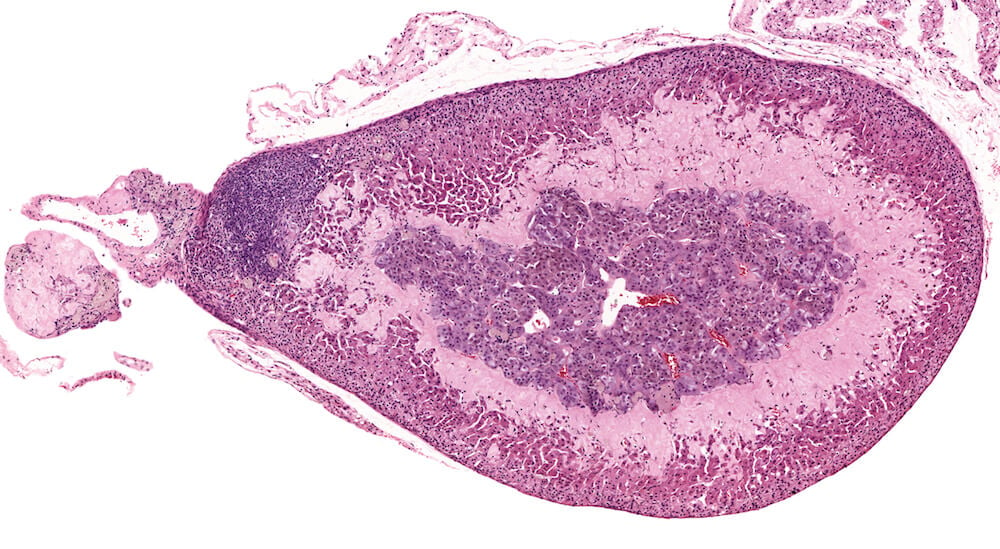
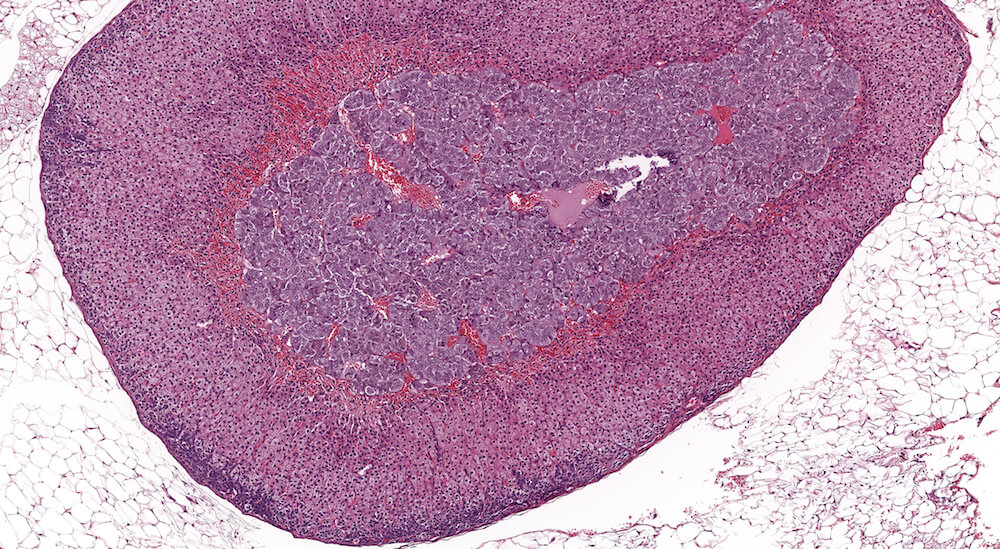
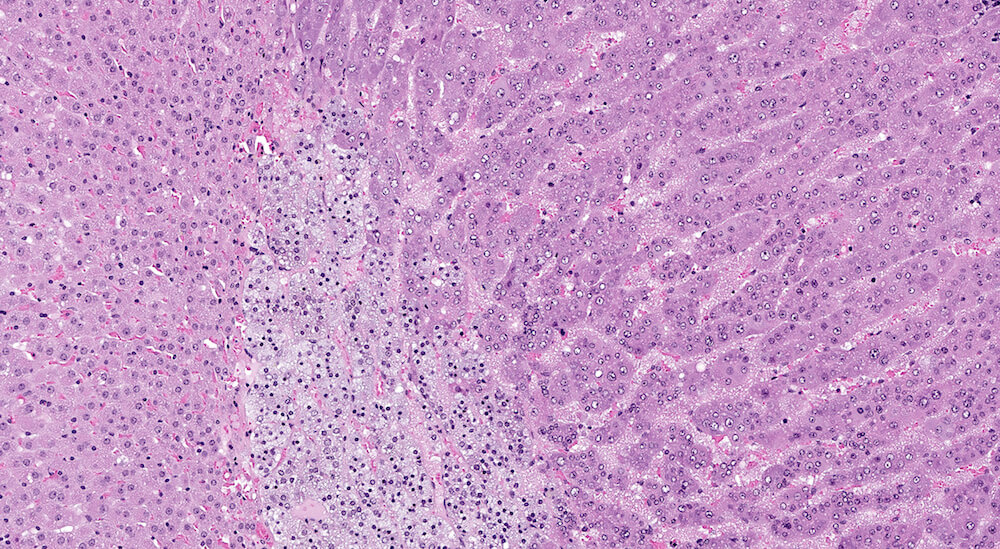
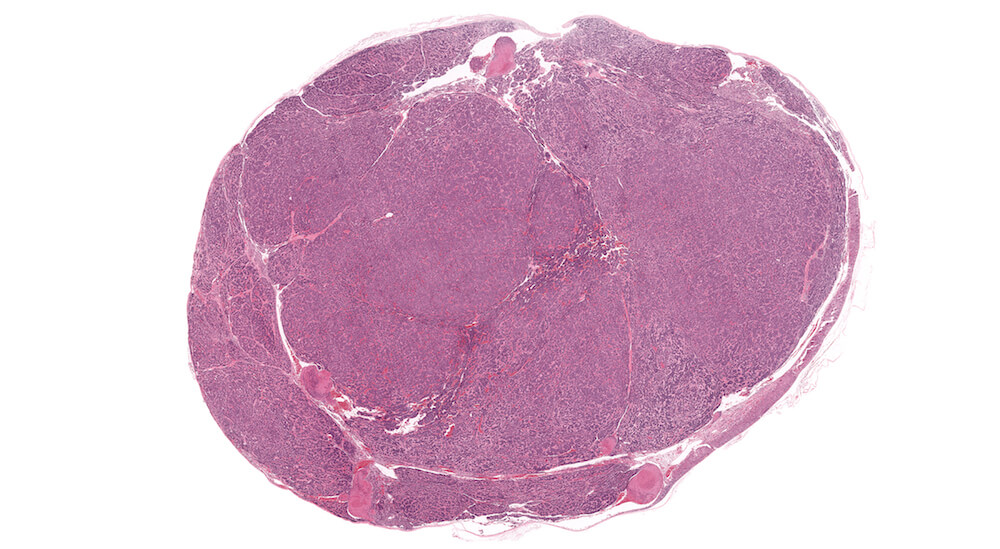
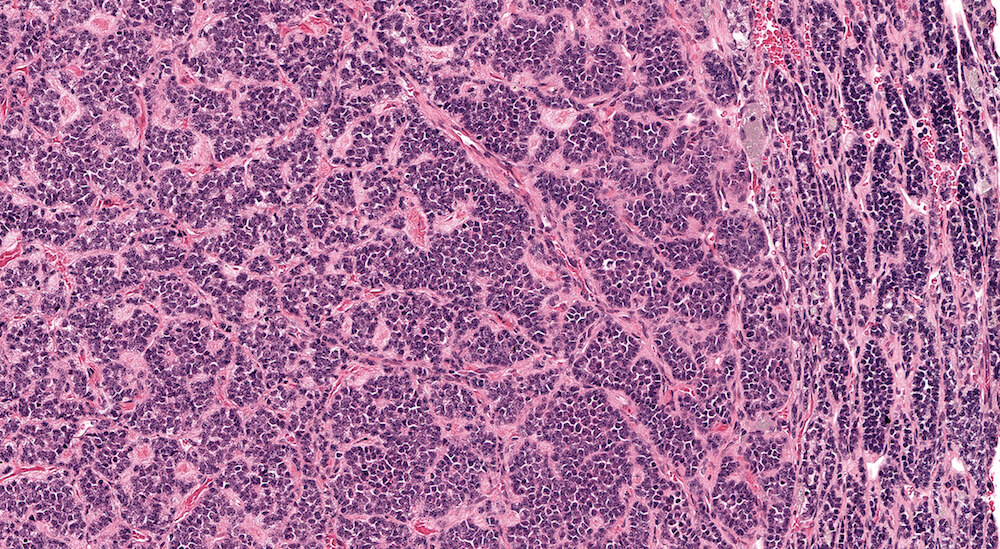
Figure 2 (A81956) Normal cortex: Control B6C3F1 male in a 90-day study. The male adrenal cortex consists of the zona glomerulosa and zona fasiculata. The zona glomerulosa is a narrow poorly delimited band of nest of cells with round nuclei and scant cytoplasm located immediately below the capsule. The junction with the zona fasiculata is often difficult to clearly define as in this case. The zona fasiculata cells are polygonal to round with a centrally located nucleus and are arranged in single cell columns perpendicular to the capsular surface with fine capillaries between adjacent columns. The columnar arrangement is less obvious near the corticomedullary junction. Varying degrees of cytoplasmic vacuolation reflecting steroid synthesis and storage are present in the zona fasiculata. In this control male mouse from a 90-day toxicity study, the degree of cortical cytoplasmic vacuolation is within normal limits and would not typically be diagnosed and graded in a conventional toxicology study. Some degree of zona fasciculata cytoplasmic vacuolation is expected as a reflection of the circadian metabolic status of steroidogenesis.

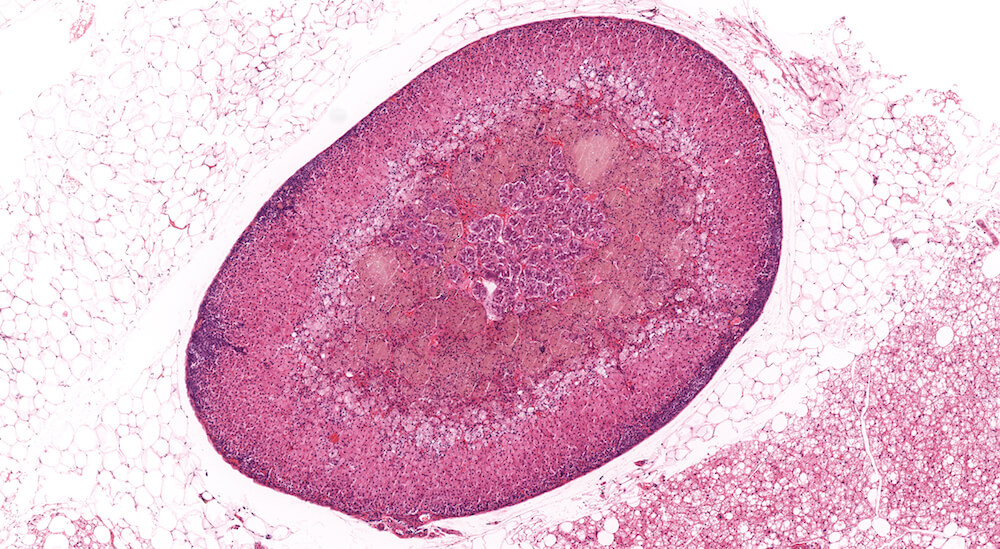
Figure 3 (A81845). Normal adrenal: Control B6C3F1 male in a chronic study. In comparison to Figure 2 (A81956), the zona glomerulosa is more readily apparent and comprised of nests of cells with pale staining cytoplasm beneath the capsule. In this adrenal the columns of the zona fasiculata are less distinct in comparison with Figure 2 (A81956). A rich capillary network is present in the cortex and medulla. Also, the degree of cortical cell vacuolation is greater than in Figure 2 (A81956) and involves both zona glomerulosa and zona fasciculata.
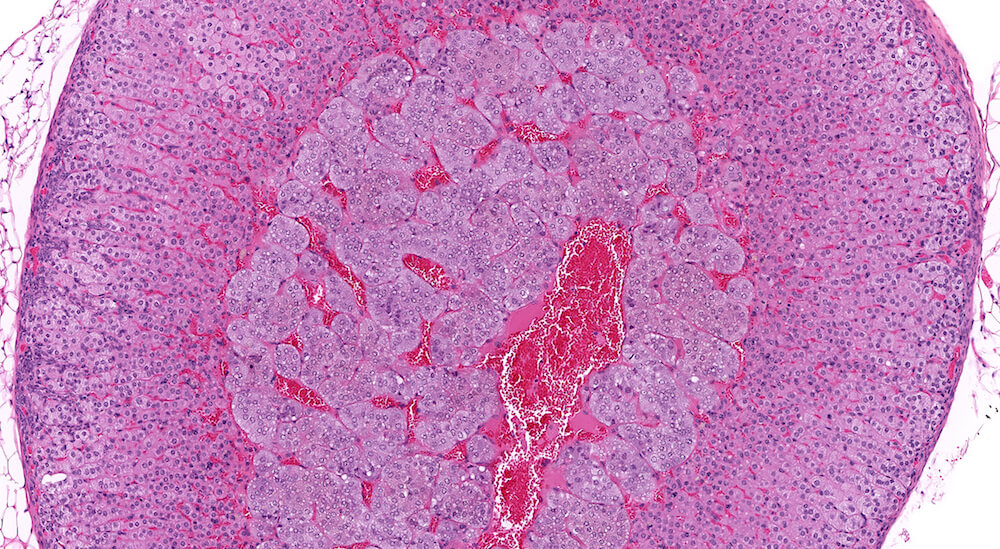
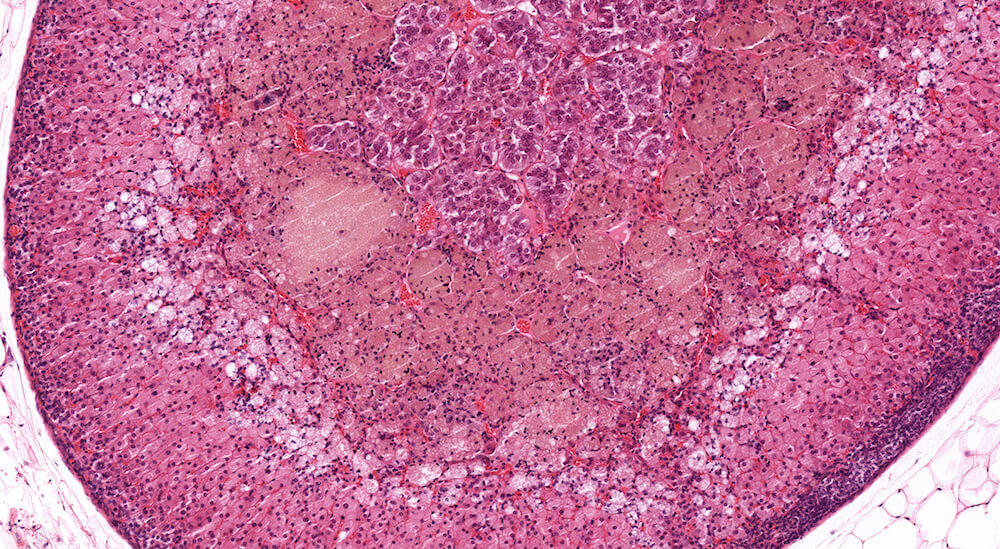
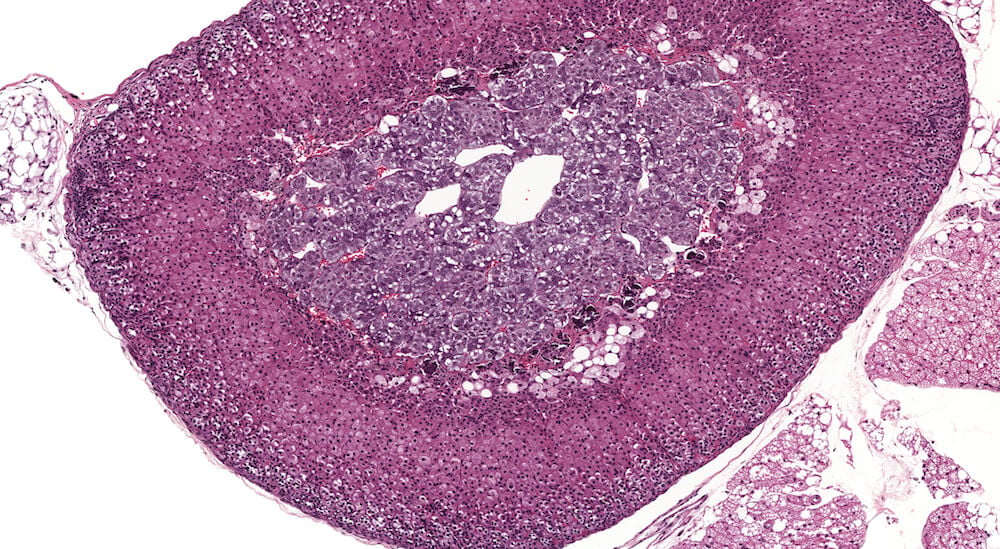
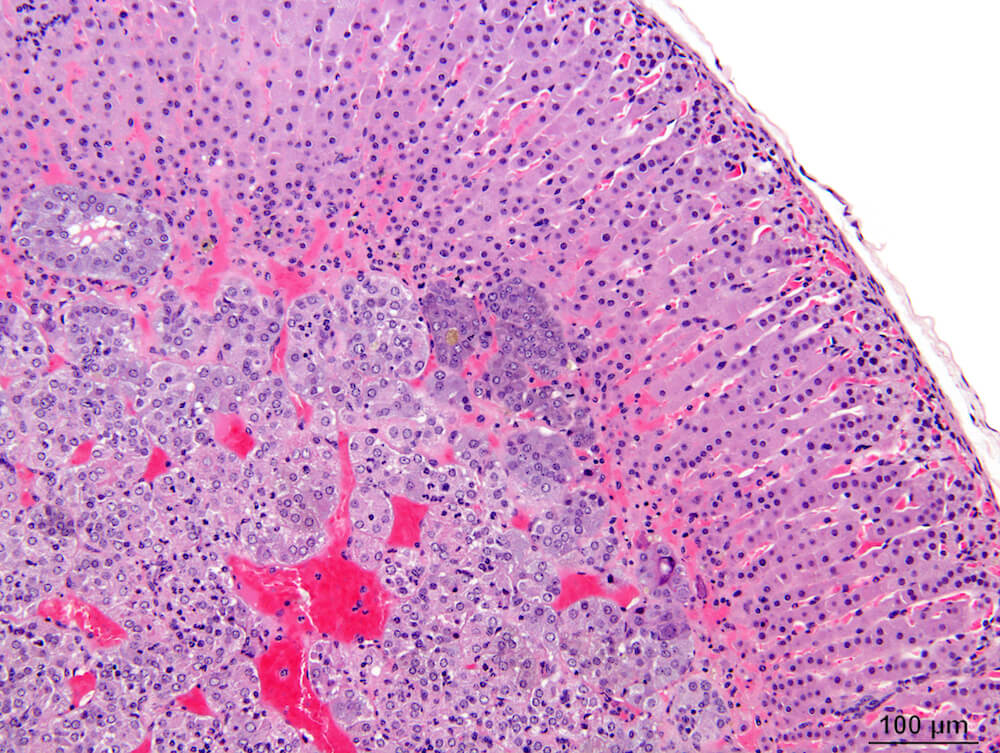
Figure 4 (A81846). Normal adrenal: Control B6C3F1 male in a chronic study.
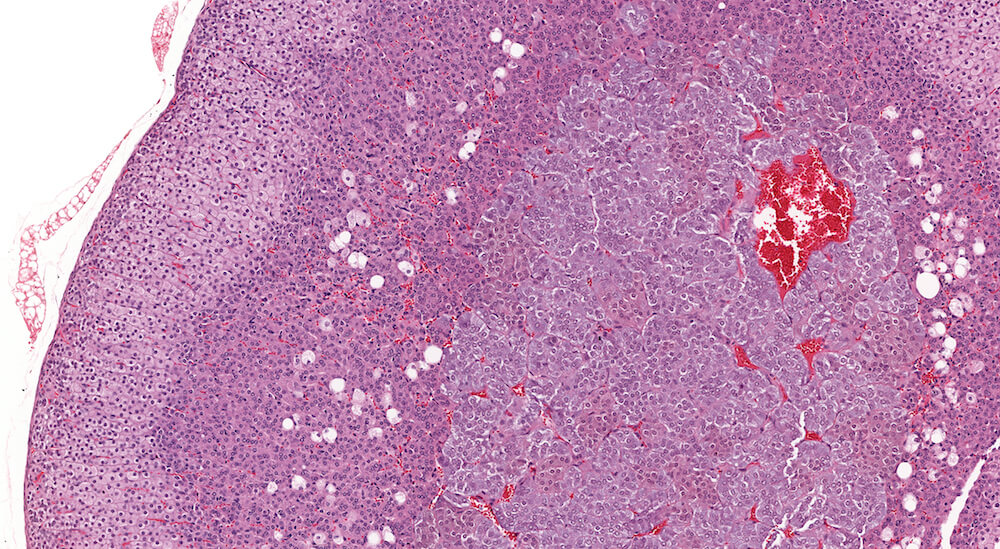
This is the contralateral adrenal in the same mouse as Figure 3 (A81845) and shows the extensive vascular system in the medulla. The subtle subcapsular cellular proliferation evident by closely packed basophilic nuclei is a normal occurrence in older mice. This degree of subcapsular hyperplasia is minimal and is an expected background change. It would typically not be diagnosed in evaluating a study unless there was a need to establish a grade for comparison to a potential treatment related response in mice exposed to a test agent or procedure. A small cluster of cells in the zona fasciculata consists of cells that are larger and more vacuolated than the surrounding zona fasciculata. These cells presumably have stored more cortical hormone but the reason for such a focal change is uncertain.
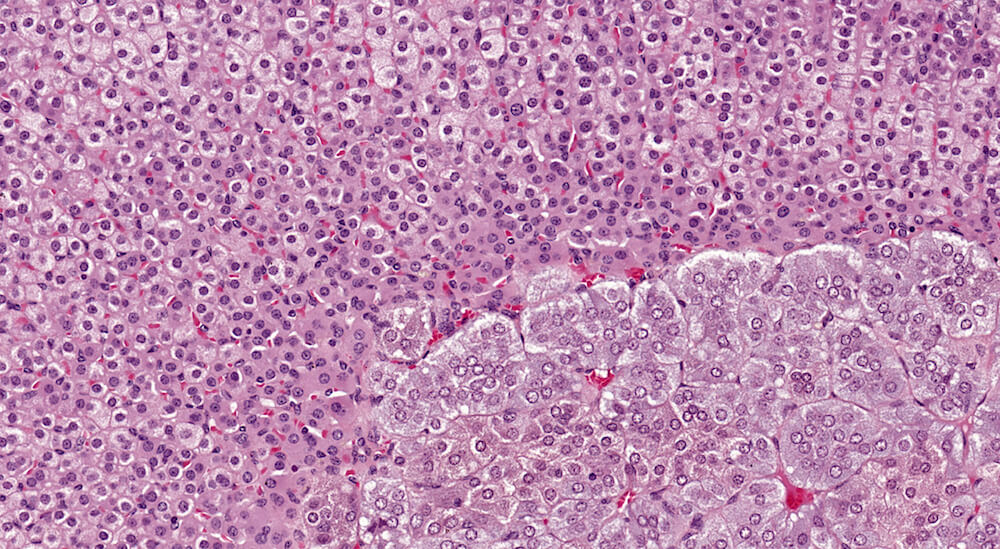
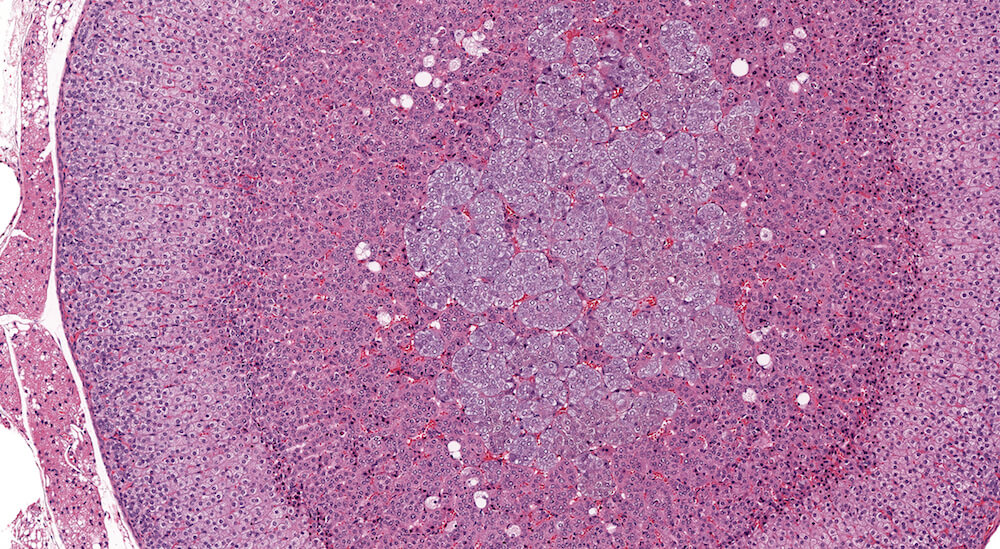
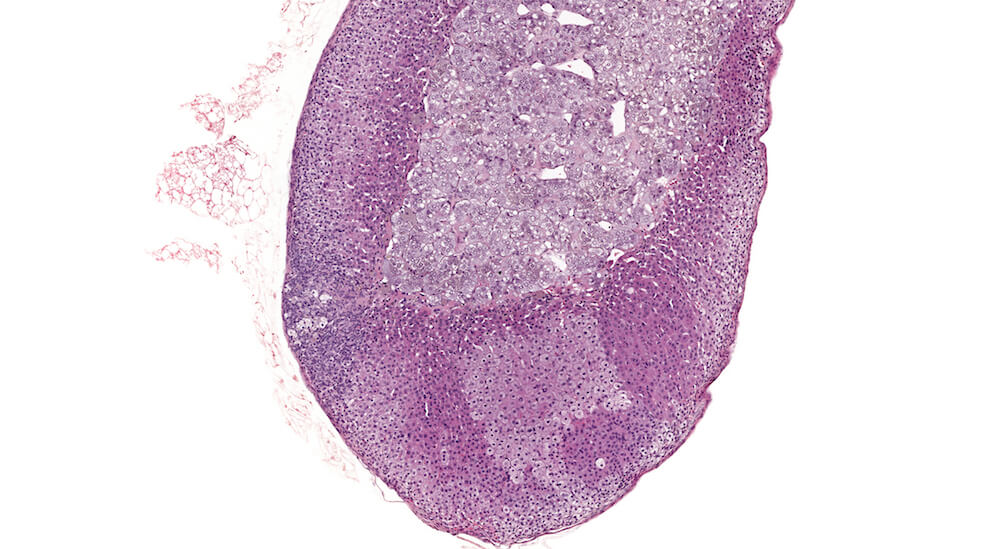
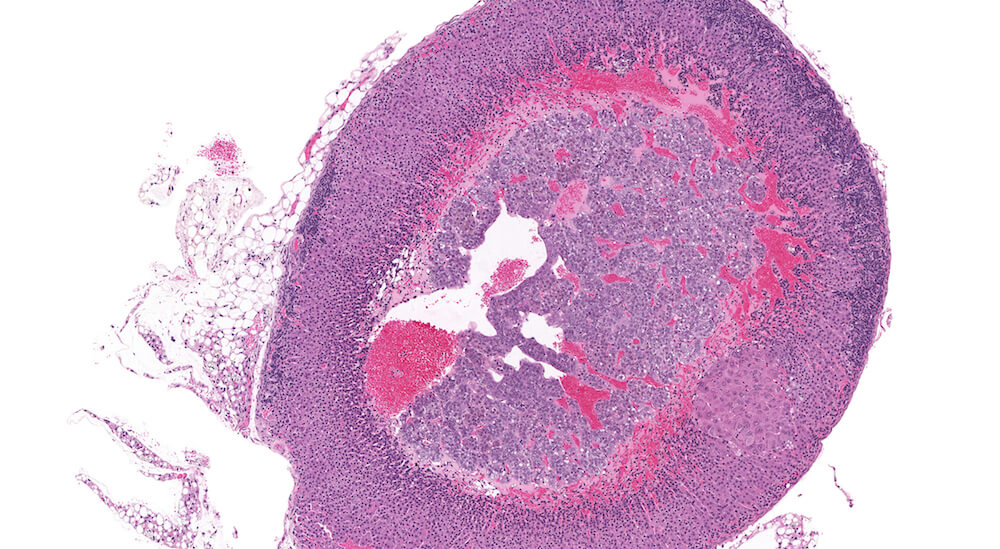
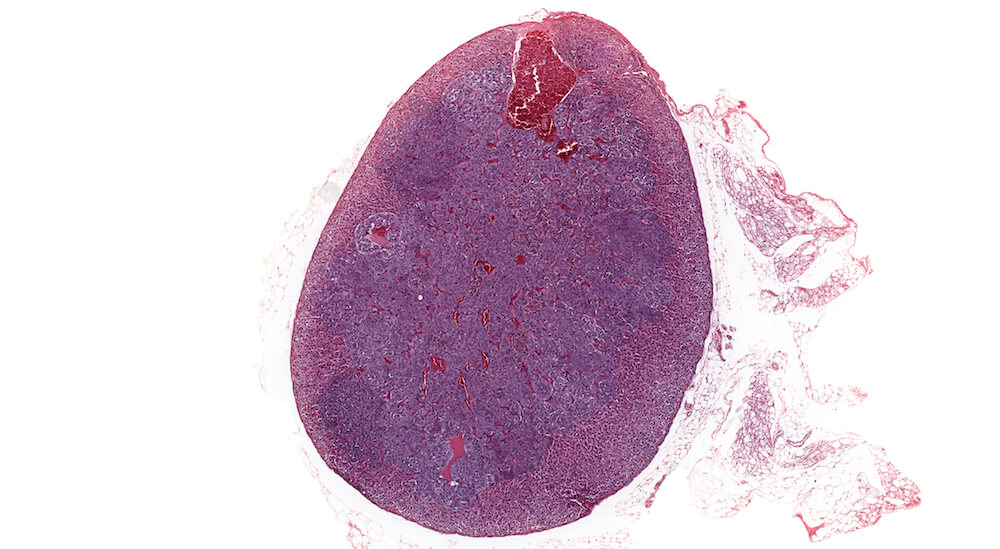
Figures 5a and 5b (A81973 & A81974). Normal adrenal. Adrenal from a normal male B6C3F1 mouse in a 90-day study. The cortex and medulla are normal and the low magnification of the adrenal (Figure 5a) shows a subtle variation in staining within the zona fasciculata. The paler staining areas of the zona fasciculata have slightly enlarged cells with finely vacuolated cytoplasm consistent with storage of cortical hormone. Present in the low magnification and more clearly in the higher magnification (Figure 5b) is the absence of an x-zone characteristic of the male mouse adrenal at this age. The normal medulla is characterized by small nests and packets of medullary cells.
Figures 6a and 6b (A81899 & A81900). Normal X-zone. Untreated female B6C3F1 mouse in a 90-day study. At the end of a 90-day study female B6C3F1 mice would be approximately 18 weeks old with a prominent adrenal x-zone as shown in right and left adrenal glands in this mouse. A small number of vacuolated cells are present within this x-zone but their presence or absence may vary among control female mice. Since this x-zone is within normal limits for this age mouse, a specific diagnosis is not necessary but its extent and appearance should be considered when evaluating possible accelerated x-zone involution in treated study cohorts. It is noted that the volume of the x-zone may vary depending a cross-section (Figure 6a) versus a more longitudinal section (Figure 6b).
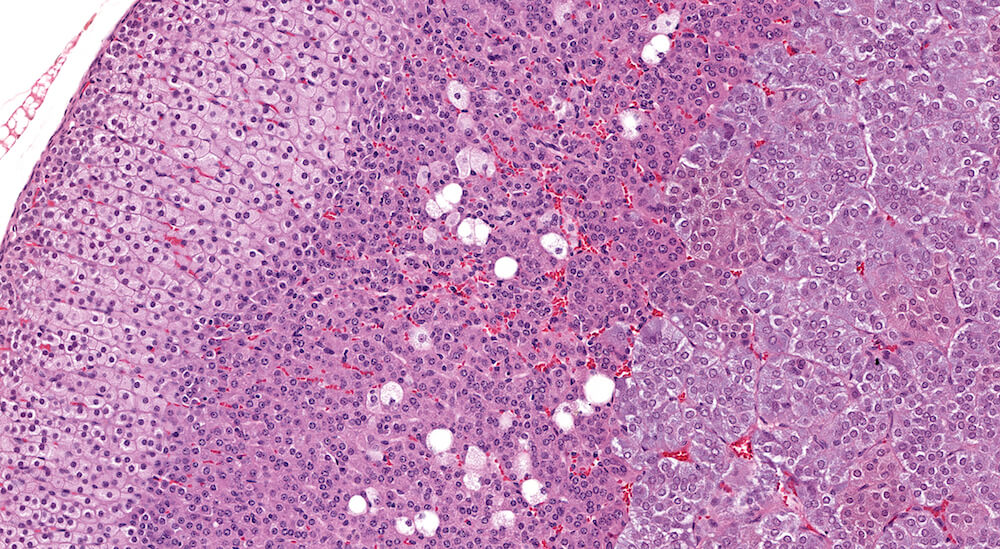
Figure 7a and Figure 7b (A81923 & A81924). Normal x-zone: Control female B6C3F1 mouse in a 90-day study. The x-zone contains a mild scattering of vacuolated cells consistent with fatty change. X-zone fatty change in normal female B6C3F1 mice approximately 18 weeks of age is highly variable. This amount of x-zone fatty change is slightly greater than in Figures 6a and 6b. The degree of vacuolation of zona fasciculata cells is within normal physiological limits.
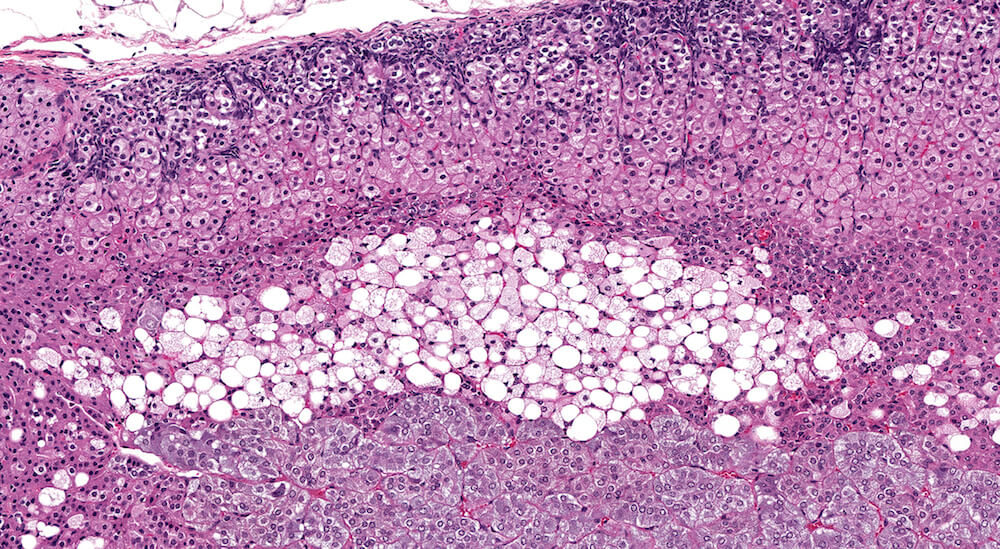
Figures 8a & 8b (A81920 & A81921). Normal x-zone with cytoplasmic vacuolation: Untreated female B6C3F1 mouse in a 90-day study. Prominent cytoplasmic vacuolation is present in this x-zone representing a more extreme example of vacuolar change sometimes present in adrenal glands in untreated 18-week old female mice (see Figures 6a, 6b, 7a, and 7b for comparison). Since this degree of cytoplasmic vacuolation is part of the variable spectrum of x-zone changes in 18-week old female mice, it need not be diagnosed but because of its extent it could be described in the study pathology narrative. While some pathologists have considered a diagnosis of fatty degeneration for this feature of the x-zone in a control of this age, this feature of the x-zone is a normal part of natural x-zone involution. It should be kept in mind that x-zone vacuolation (fatty change) is a non-exclusive form of normal x-zone involution. Involution can occur without any evidence of vacuolation (fatty change). A minimal to mild degree of subcapsular hyperplasia is present in this adrenal gland.
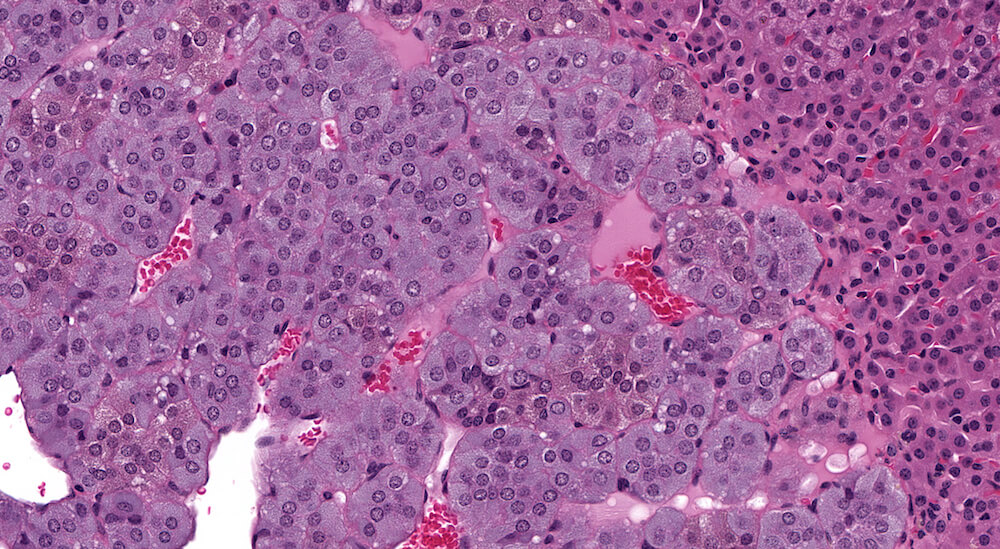
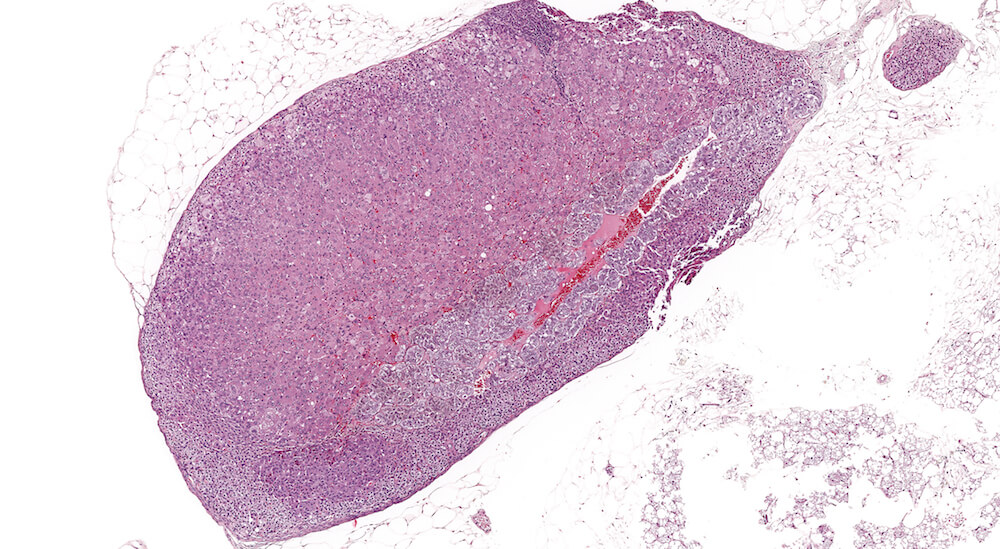
Figure 9 (A81958). Normal medulla: Control B6C3F1 male in a 90-day study. The adrenal medulla is comprised of nests of cells with closely spaced leptochromatice nuclei and finely granular slightly basophilic cytoplasm. The cellular nests are separated by intervening capillaries and larger vascular channels that provide a rich vascular network draining blood from the cortex. Although not apparent in this example, subtle cytoplasmic tinctorial variations may allow identification of epinephrine versus norepinephrine medullary cells. Some cortical cells at the corticomedullary junction have condensed hyperchromatic as well as pyknotic nuclei reflecting the final stages of normal cell death of cortical cells. Dying cell fragments are most likely carried away via the vascular system.
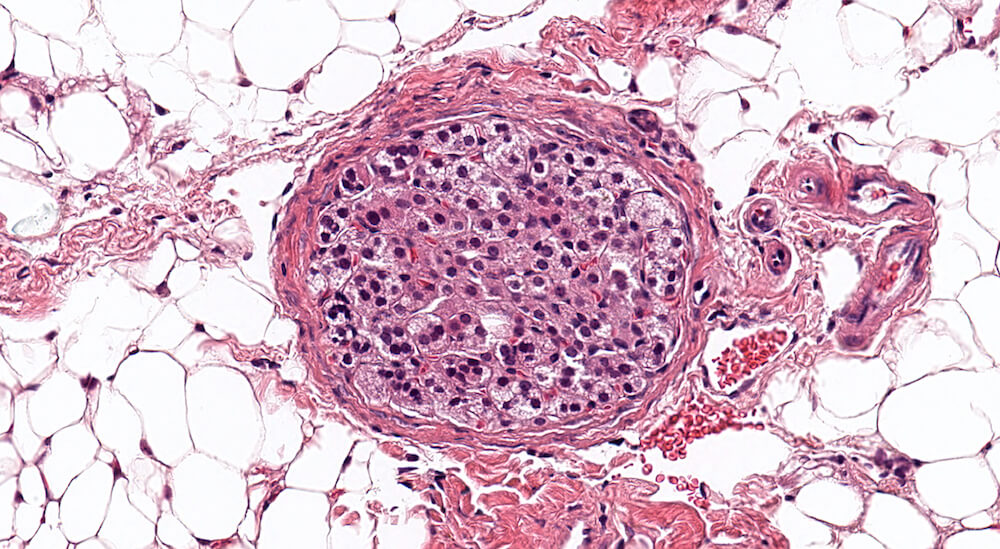
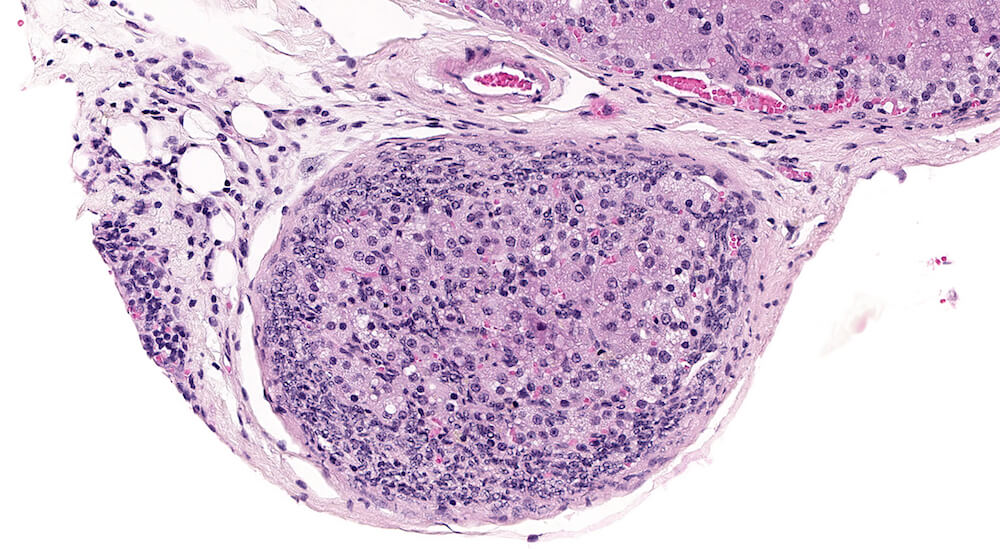
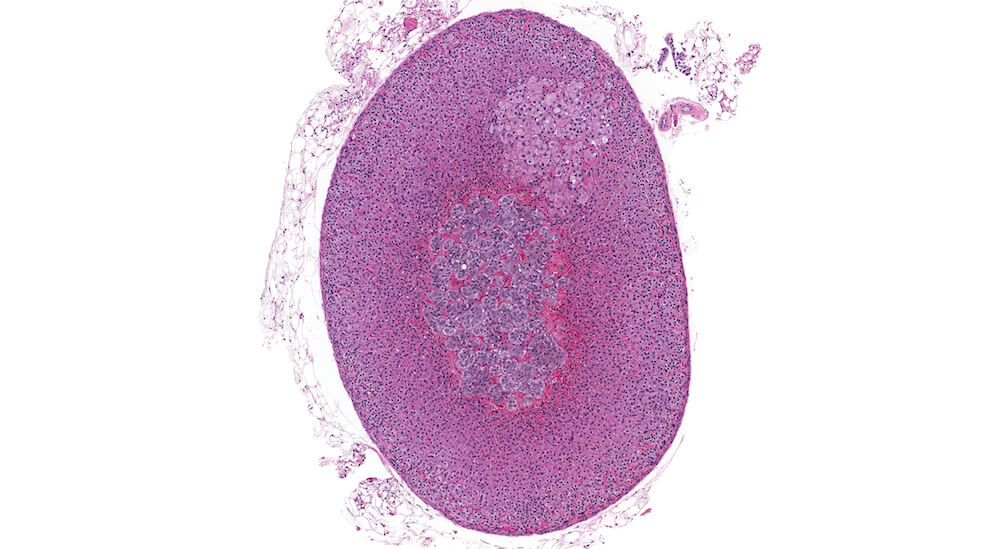
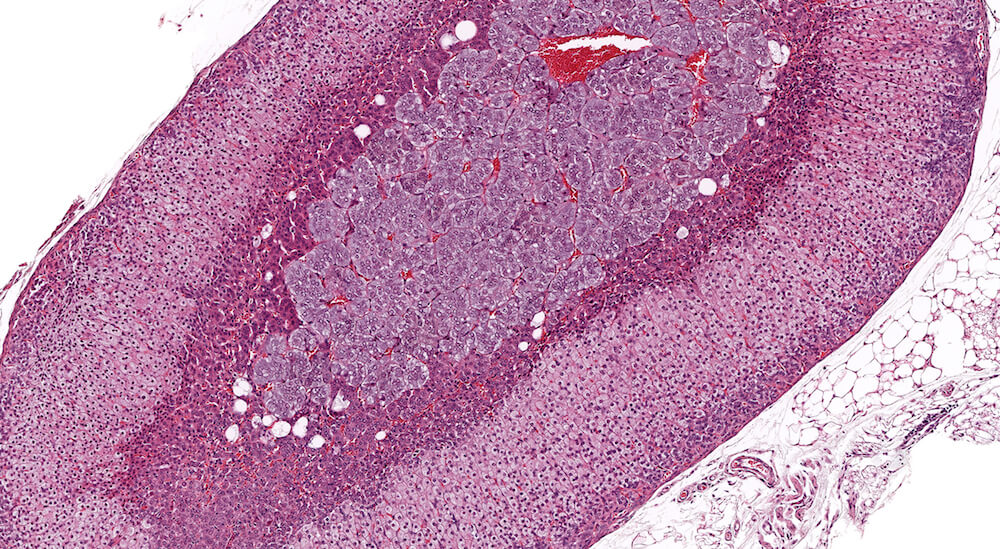
Figures 10 (A81904), 11 (A81867) & 12 (A81948). Accessory cortical nodule: Figures 10 and 11 are untreated controls and Figure 12 is a treated B6C3F1 mouse. All are males. Accessory cortical nodules are relative common in both male and female mice, have a strain-dependent variable incidence, and may be attached to or just beneath the adrenal capsule or separate in adjacent periadrenal adipose tissue. The consist of both zona glomerulosa and zona fasciculata cells and are generally diagnosed even though their incidence does not appear to be influenced by treatment.
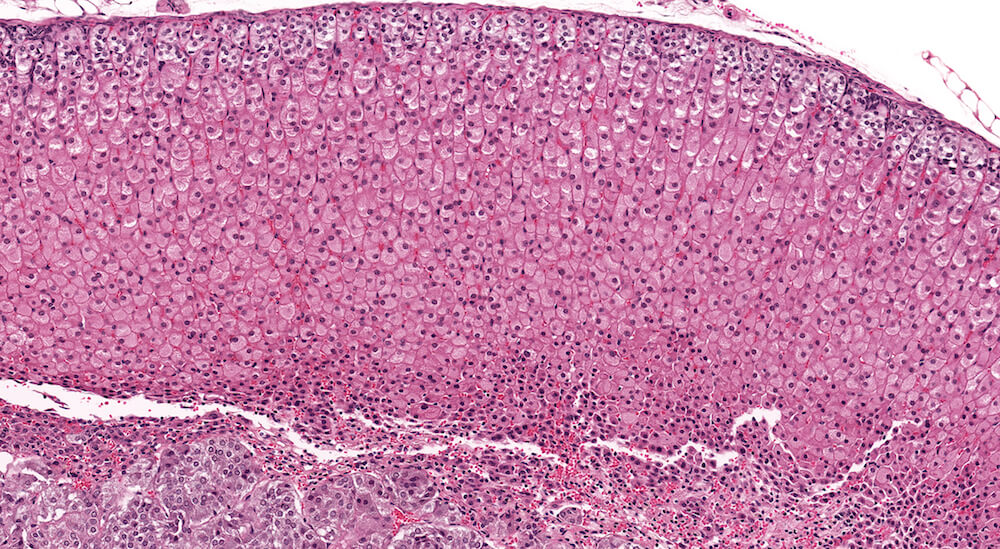
Figure 13 (A81975). Diffuse cortical hypertrophy: Treated B6C3F1 female in a 90-day study. There are two notable changes present in this adrenal. The zona fasiculata is comprised of uniformly enlarge cells is finely vacuolated cytoplasm. This change is consistent with storage of steroid hormone or precursors and was originally diagnosed as increased cytoplasmic vacuolation. However, it could also represent a physiological change rather than a pathological process; thus, a diagnosis of increased cytoplasmic vacuolation should be based on comparison to age-matched concurrent controls. Conclusions regarding the importance of this type of change can be supported by significant increase in adrenal weight. The second notable change in this adrenal gland is a narrowing of the x-zone associated with increased nuclear density and minimal congestion. Since this is a 90-day study in a female mouse, the X-zone should normally be much wider (see Figure 6a for comparison). Consequently, this change represents premature x-zone involution and should be diagnosed. Premature involution of the x-zone may occur as a direct effect of treatment or as a hormonal andromimetic response.
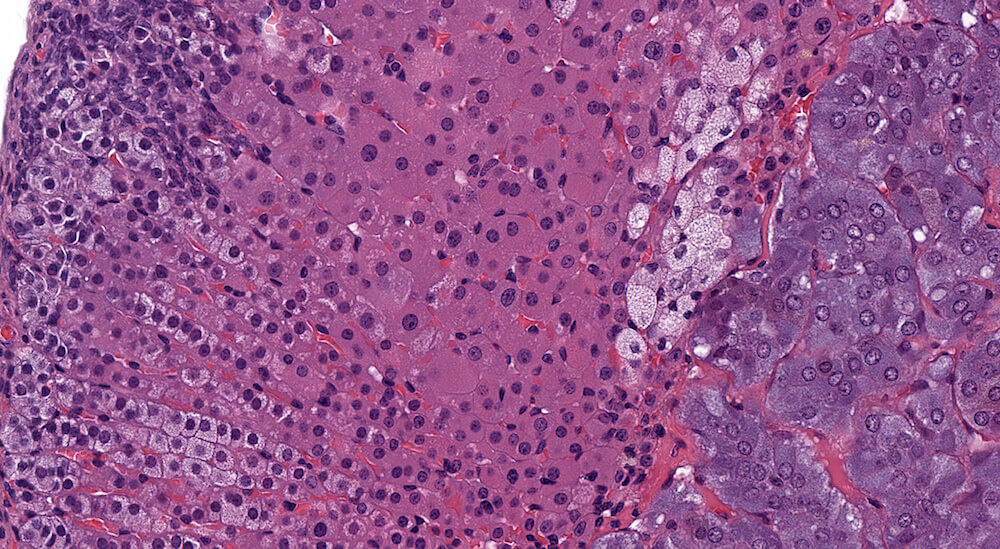
Figures 14a (A81888), 14b (A81891) & 14c (A81890). Focal cortical hypertrophy: Vehicle control B6C3F1 male in a chronic study. In addition to presence of subcapsular hyperplasia (Figures 14a & 14b), there is an irregular patch of enlarged zona fasiculata round to polyhedral cells with uniformly stained, eosinophilic finely granular cytoplasm and centrally located nuclei (Figures 14b & 14c). Nuclei in this focus of hypertrophic cells vary in size suggesting some degree of polyploidy (Figure 14c). The function of this focus of hypertrophic cells is uncertain but may reflect a localized area of hormonal synthesis. A small irregular collection of enlarge cells with pale staining stipulated cytoplasm and somewhat condensed nuclei between the focus of hypertrophy and the medulla suggests early degeneration. In evaluating a toxicity study, the cytomorphological features of the hypertrophic cortical cells warrants description in the pathology narrative.
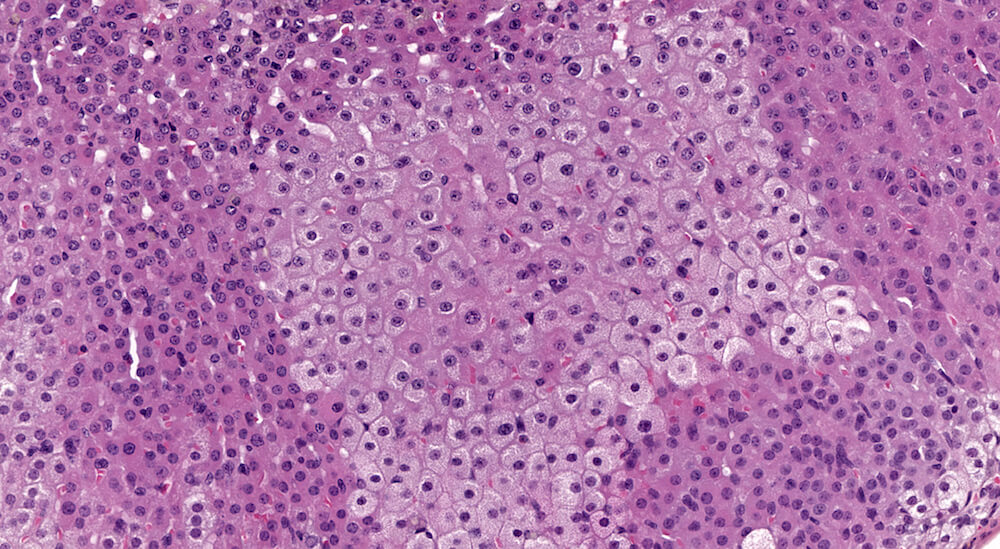
Figures 15a (A81873) & 15B (A81875). Focal cortical hypertrophy: Treated B6C3F1 male in a chronic study. A sharply delimited approximately square focus of pale staining hypertrophic cells is located in the zona fasiculata. Hypertrophic cells have a finely vacuolated cytoplasm with centrally located nuclei in this unusually shaped lesion. There is no apparent compression of the smaller adjacent zona fasiculata cells. Depending upon one’s diagnostic lexicon, this change could be diagnosed as focal cortical hypertrophy or alternatively as focal cytologic alteration. In either case a description of the cellular features would be an appropriate addition to the pathology narrative. The functional significance of this change is uncertain. A focus of subcapsular hyperplasia is also present in this adrenal (Figure 15a).

Figures 16a (A81880) & 16b (A81881). Focal cortical hypertrophy: Low dose B6C3F1 male in a chronic study. A discrete focus of cellular alteration consisting of pale pink staining cells is present in the zona fasiculata (Figures 16 a & 16b). The cellular features of focus cells at high magnification include sizes ranging from crowded small cells mixed in with very large cells, cytoplasm that is finely granular with a more open texture compared to cytoplasm of adjacent zona fasiculata parenchyma, and variably sized nuclei. There are occasional cytoplasmic vacuoles in the focus cells and some cells have pyknotic nuclei. The variable nuclear morphology in this focus suggests some degree of polyploidy in contrast to hypertrophic cell in Figure 15b (A81875).
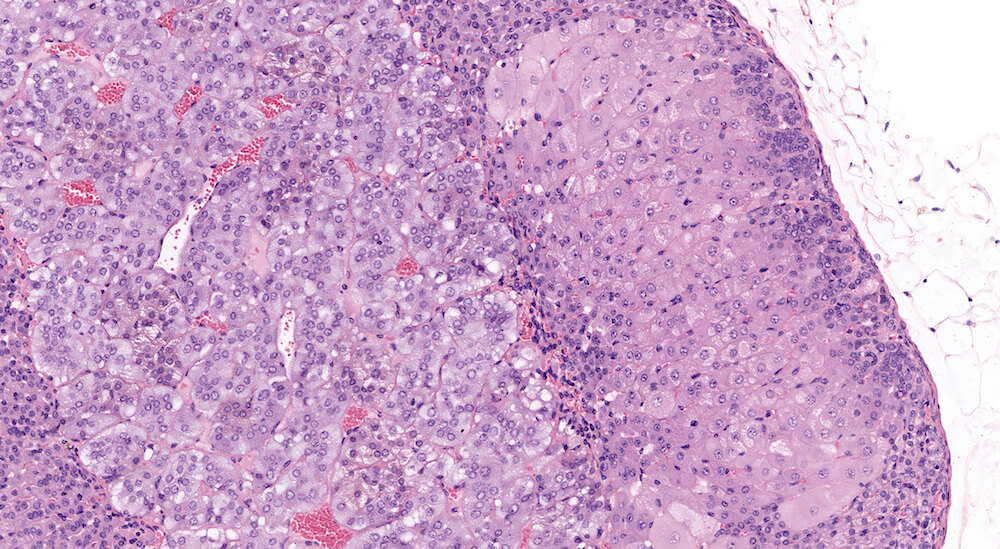
Figures 17a (A81855) & 17b (A81858). Focal cortical hypertrophy: Vehicle control male B6C3F1 mouse in a chronic study. The focus of hypertrophy is comprised are enlarge cells with eosinophilic cytoplasm (Figure 17b) with mild distortion of the normal contour of the adrenal cortex (Figure 17a). The functional significance of focal hypertrophy is unclear but suggests localized increased storage of cellular material related to steroidogenesis. There was no cortical hypertrophy in the contralateral adrenal. A small focal area of subcapsular hyperplasia is adjacent to the focal hypertrophy (Figure 17a).
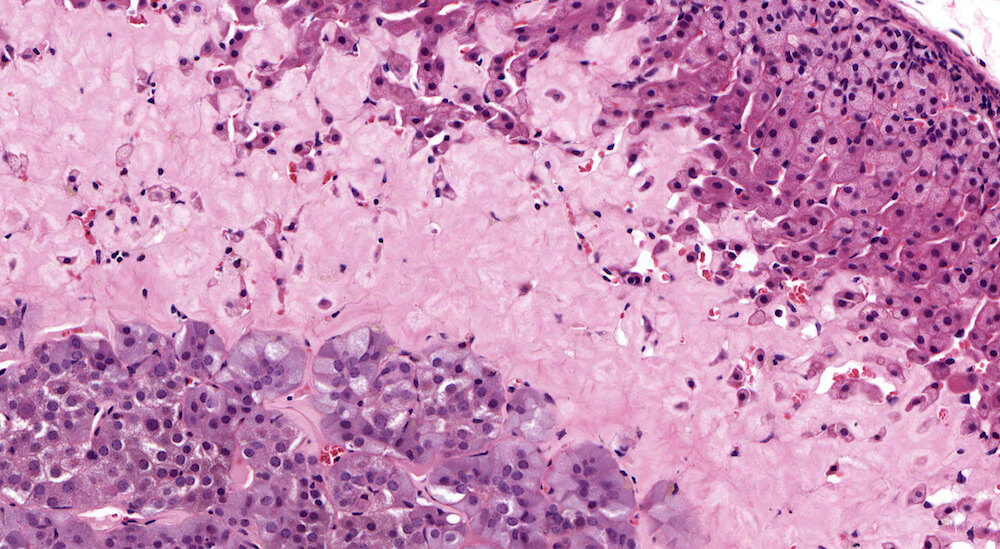
Figure 18a (A81971) and Figure 18b (A81972). Amyloidosis: Treated Swiss Webster male in a chronic study (need age). A band of homogeneously staining pink material consistent with amyloid extends from the mid-zona fasiculata to the corticomedullary junction (A81971 & A81972). Amyloidosis is strain and age dependent and represents deposition of insoluble hydrophobic fibrils of misfolded protein. It is generally a systemic response affecting multiple organs, has been reported to be more frequent in females and associated with fighting among group housed males.
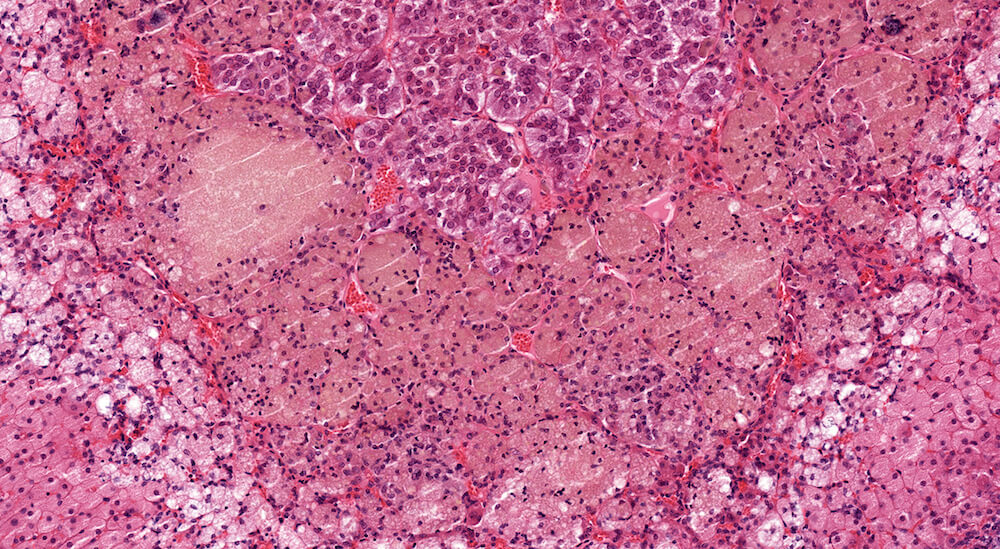
Figures 19a (A81953), 19b (A81954), & 19c (A81955). Lipogenic pigment: Treated female B6C3F1 mouse in a chronic toxicity/carcinogenicity study. Vacuolated cells in the x-zone at the corticomedullary junction are present along with variably sized clusters of lipid-laden epitheloid cells containing lipogenic pigment (Figures 19a & 19b). The pigment deposition is central to but closely associated with the x-zone and extends into the medulla (Figures 19b & 19c). Lipogenic pigment is an age-associated degenerative change in mice and can be exacerbated by different treatments. Multiple areas of minimal to mild subcapsular hyperplasia are present in the adrenal cortex (Figures 19a & 19b7).
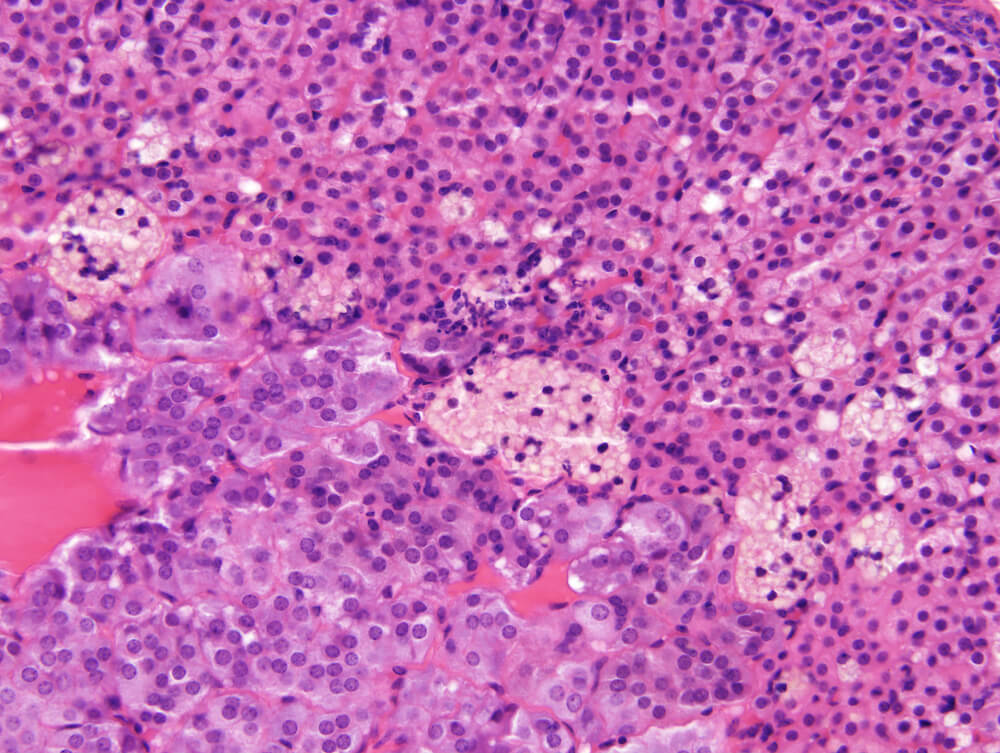
Figures 20a (A54950) & 20b (A54951). Lipogenic pigment: A male B6C3F1 mouse from a chronic toxicity/carcinogenicity study. A band of multiple small to medium sized clusters of enlarged cells filled with foamy, lightly staining yellowish-brown pigment is present in the inner zona fasciculata and extends to the corticomedullary junction. The pigment containing epitheloid cells have pyknotic nuclei (Figure 20a). At higher magnification (Figure 20b) a few individual pigment-containing cells are present in the zona fasciculata. Since this is a male mouse whose x-zone underwent complete involution early in life at sexual maturity, the lipogenic pigment most likely originates from breakdown of cytoplasmic steroidal hormone or hormone precursors.
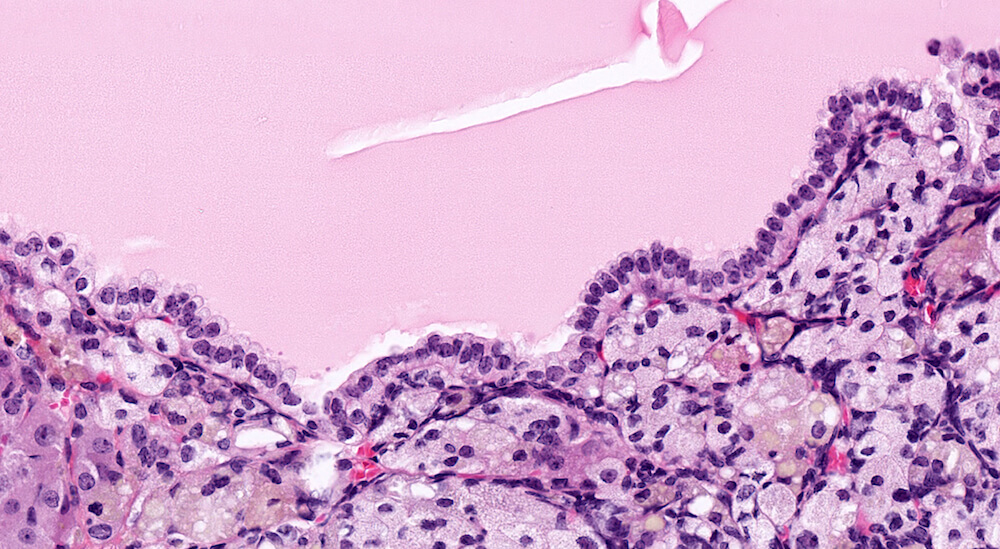
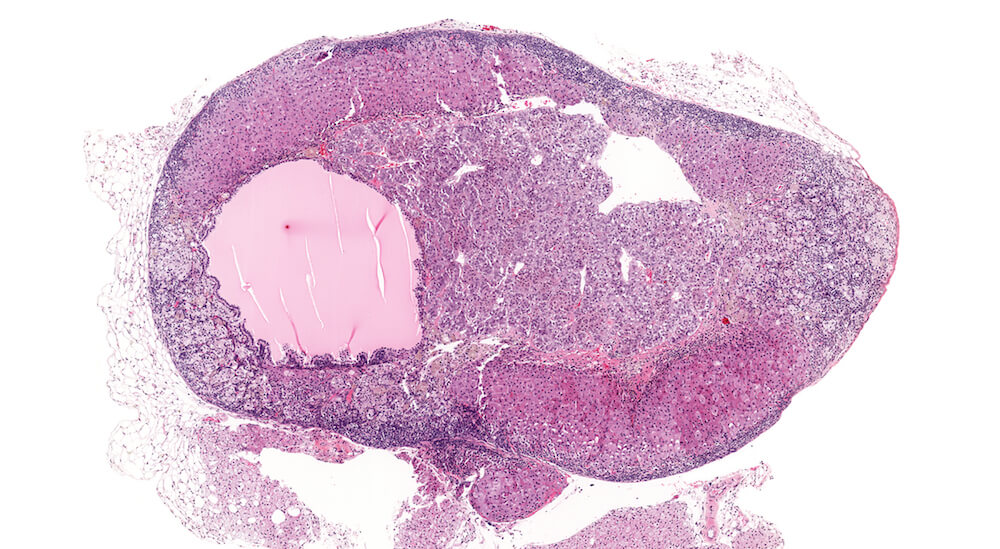
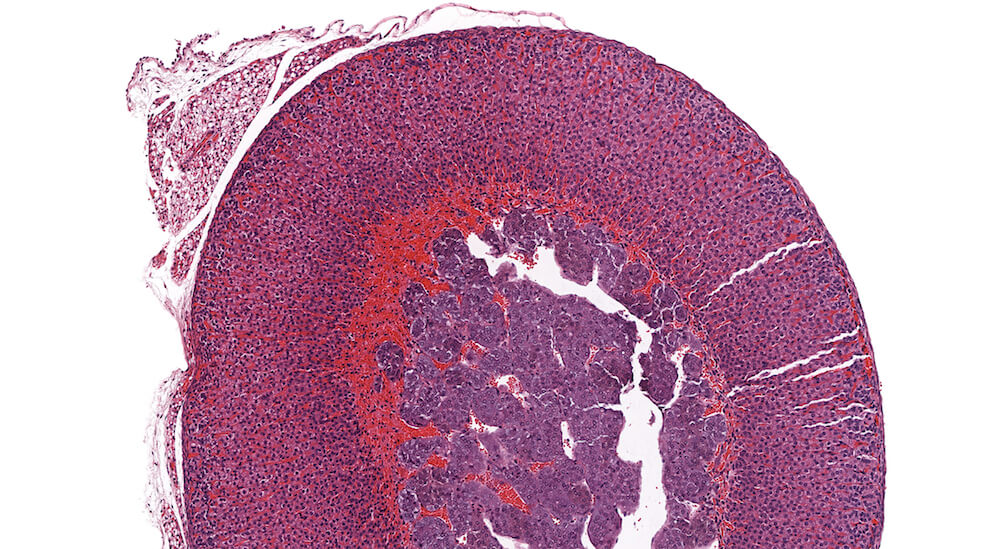
Figure 21a (A81937) & 21b (A81938). Cortical cyst: Treated female B6C3F1 in a chronic study. A large fluid-filled cyst is present in the cortex causing compression especially noted in the medulla Figure 21a). This cyst is lined by a single layer cubidal to taller cuboidal cells (Figure 21b) but other cysts may be lined by columnar ciliated epithelium. Cortical cysts are rare and probably represent a developmental alteration. It is recommended to diagnose cysts during the evaluation of toxicity or experimental studies. There is a diffuse subcapsular hyperplasia consisting of type A as well as type B subcapsular cells. Subcapsular hyperplasia is a common aging change that is not related to the development of the cortical cyst. This degree of subcapsular hyperplasia warrants diagnosis during study evaluation.
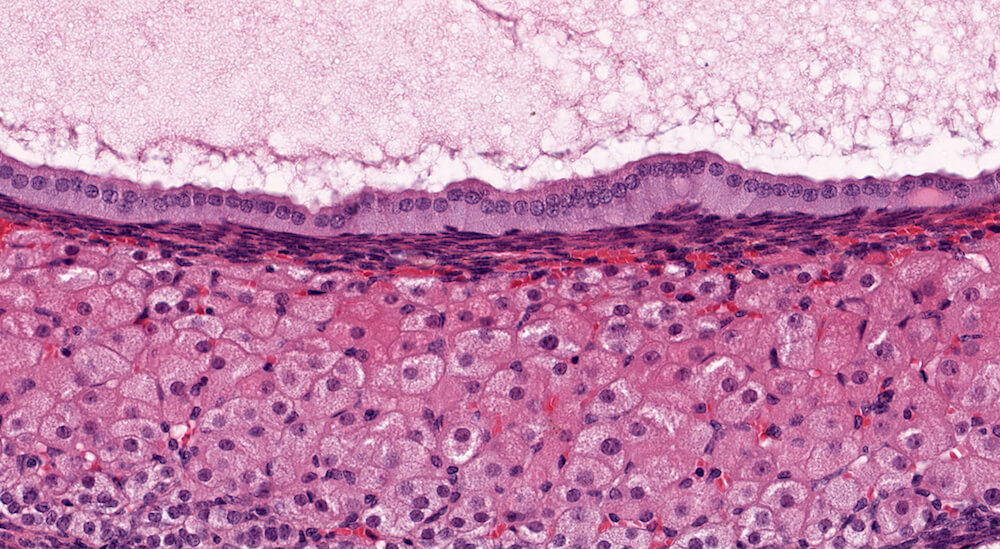
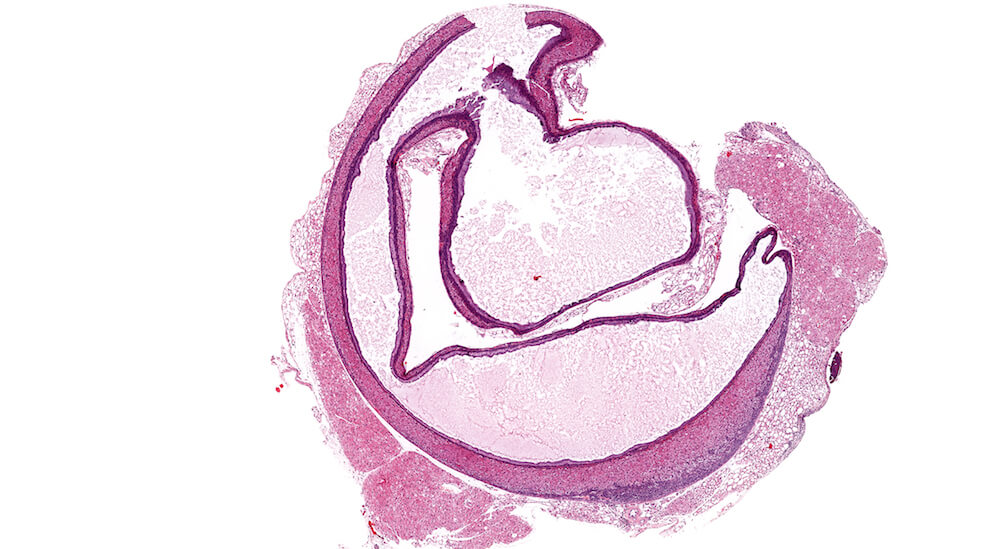
Figure 22a (A81939) & 22b (A81940). Cortical cyst: Treated female B6C3F1 in a chronic study. A large and partially collapsed cyst lined by columnar epithelium (Figures 22a & 22b) occupies most of the adrenal gland and is delimited by a narrow band of compressed cortical parenchyma. The cyst content is a finely granular pale eosinophilic secretion. The high magnification shows the columnar cell cyst lining and the finely granular flocculent secretion. The compressed adrenal cortex has large round to polyhedral cells with abundant foamy cytoplasm (Figure 22b). Cortical cysts are rare and probably represent a developmental alteration. It is recommended to diagnose cysts during the evaluation of toxicity or experimental studies.
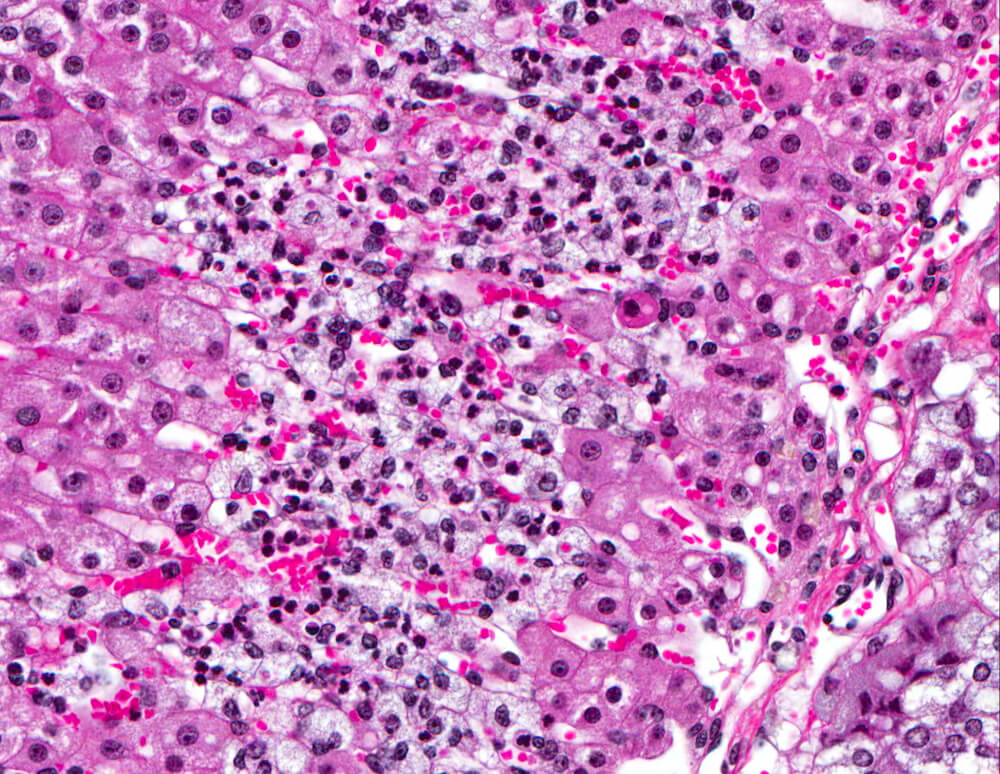
Figures 23A and 23B. Cortical inflammation: Female B6C3F1 mouse in a chronic study. A band of vacuolated and necrotic cells admixed with polymorphonuclear leukocytes is present in the mid-portion of the zona fasciculata. Primary adrenal inflammation is uncommon in mice.
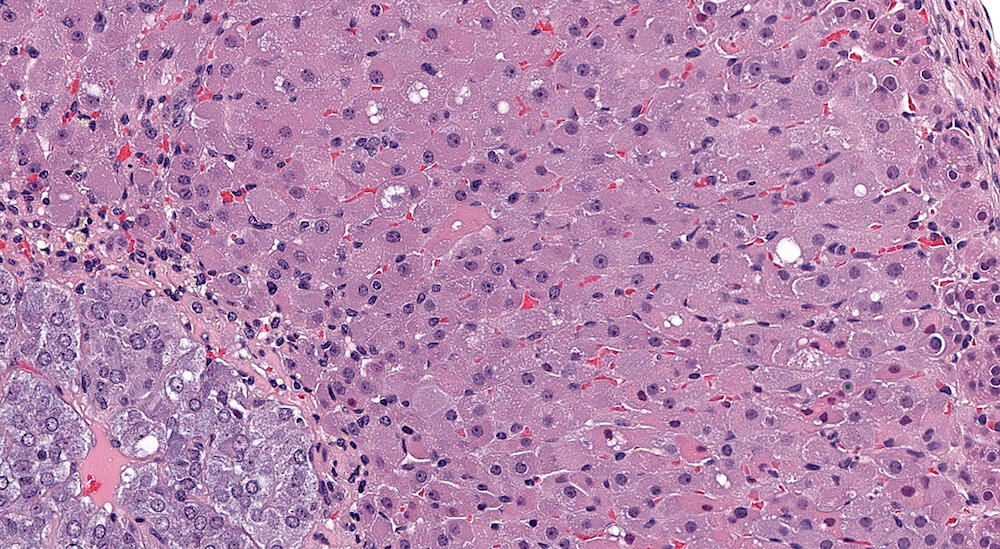
Figures 24a (A81876), 24b (A81878), & 24c (A81879). Cortical degeneration and necrosis: Treated (high dose) B6C3F1 male mouse in a chronic study. There is diffuse hypertrophy of zona fasiculata cells (Figures 24a) with pale pink and finely granular to homogeneous cytoplasm and nuclei are either leptochromatic or have condensed and hyperchromatic nuclei (Figures 24b & 24c). In the highest magnification (Figure 24c), individual cells undergoing degeneration and necrosis are present and can be identified by their slightly condensed shape, hypereosinophilic homogeneous cytoplasm, some with pyknotic nuclei, and some with cytoplasmic vacuoles. A very narrow band of compressed degenerating cells is present at the corticomedullary junction (Figure 24c).

Figures 25a (A81969) & 25b (A81970). Cortical necrosis: High-dose treated female B6C3F1 mouse in a 90-day study. A wide band of pink staining extending throughout the mid-portion of the zona fasiculata (Figures 25a & 25b) represents acute necrosis. The zona glomerulosa and outermost part of the zona fasiculata as well as the x-zone are relatively spared. Adrenal necrosis is an uncommon lesion. In this study, adrenal necrosis was seen in 5 of 10 high dose females that died before study termination. Chronic inflammation at the adrenal corticomedullary junction was present in 2 high dose females that survived to the end of the study. Necrosis was also documented the livers and lungs of high dose females.
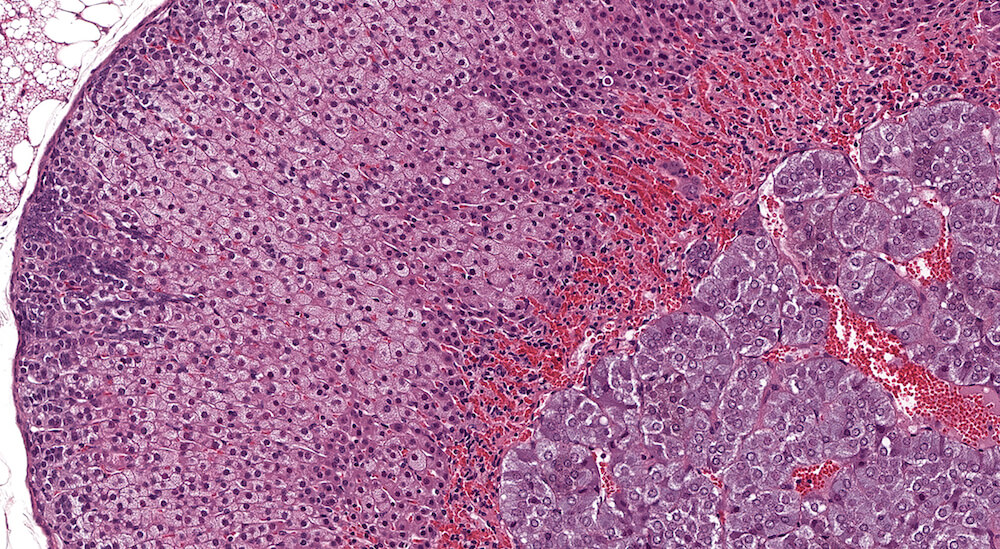
Figure 26a (A81907), 26b (A81908), 26c (A81910), 26d (A81911) & 26e (A81912). Premature x-zone involution: Female B6C3F1 mice treated with androstenedione in a 90-day study. These examples represent different degrees of X-zone involution in 18-week old female mice and are diagnosed as premature x-zone involution in contrast to the concurrent female mouse controls (see Figures 6a & 6b). The extent of x-zone involution showed a dose-response in this study reflected by a qualitative increased severity degree of premature or early involution. It is noted that premature involution in these examples is not associated with cytoplasmic vacuolation or fatty change. Mild subcapsular hyperplasia is present in these adrenals and its extent appeared to be exacerbated by treatment in contrast to concurrent controls (see Figures 6a & 6b).
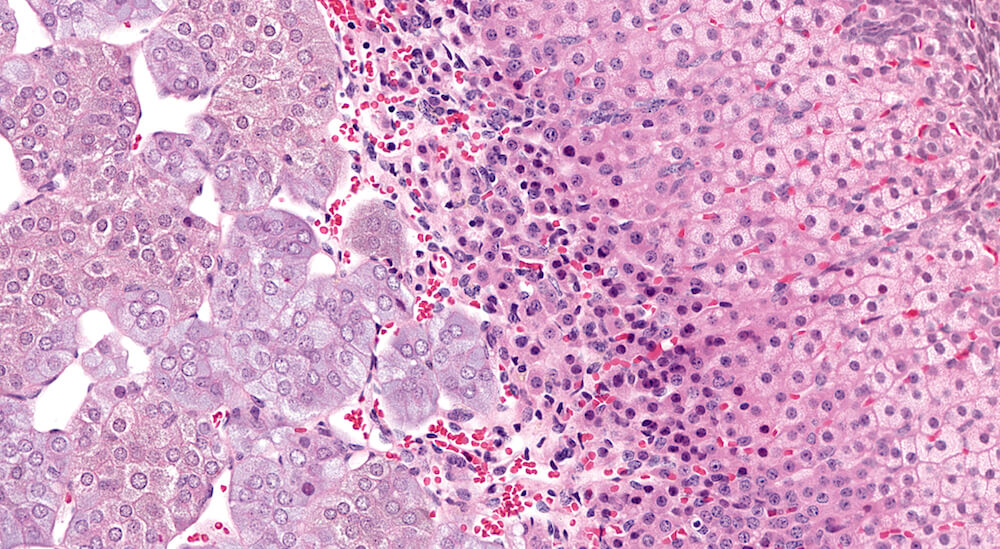
Figure 27a (A81913) & 27b (A81914). Premature x-zone involution: Female B6C3F1 mouse treated with o-nitrotoluene in a 90-day toxicity study. There is narrowing of the x-zone (Figures 27a & 27b) with presence of pyknotic nuclei admixed with more normal x-zone cells reflecting a moderate degree of x-zone involution in contrast to a concurrent study control female B6C3F1 mouse (see Figure 7b). The enlarged zona fasiculata cells have finely vacuolated pale eosinophilic cytoplasm reflecting normal storage of steroid hormone.
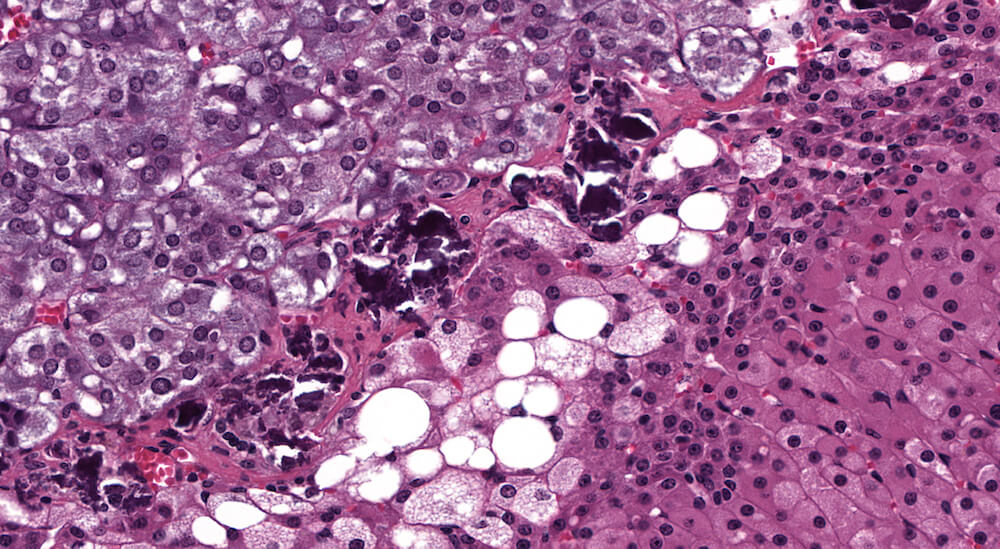
Figure 28a (A81916) & 28b (A81917). Premature x-zone involution: Female B6C3F1 mouse treated with 1-bromopropane in a 90-day study. The x-zone is markedly narrowed in contrast to similarly aged female controls (see Figures 6a & 6b) with presence of vacuolated cells (Figures 28a & 28b) consistent with fatty change and deposition of deeply stained deposits consistent with dystrophic calcification (Figure 28b). In contrast to other provided examples of premature involution, in this case involution is associated with fatty change.
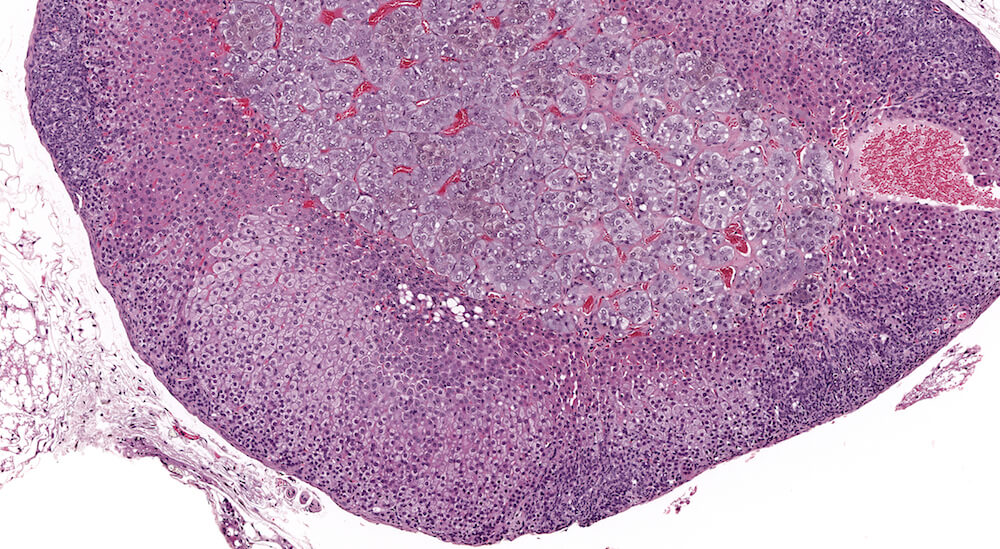
Figures 29a (A81884) & 29b (A81885). Focal subcapsular hyperplasia: Vehicle B6C3F1 male chronic study. Multiple focal areas of subcapsular hyperplasia are present in addition to a focal cortical area of cytoplasmic alteration consisting of hypertrophic vacuolated zona fasciculata cells (Figures 29a & 29b). The subcapsular hyperplasia consists of primarily small basophilic cells described in the literature as type A cells. Subcapsular hyperplasia is a common, age-related change in B6C3F1 and other mouse strains. Adjacent to the larger focus of cortical hypertrophy, there are two less discrete foci of hypertrophy (Figure 29b). Hypertrophic cells have pale staining finely vacuolated cytoplasm consistent with steroidal storage. A small collection of vacuolated cells consistent with fatty change is present at the corticomedullary junction (Figure 29b).
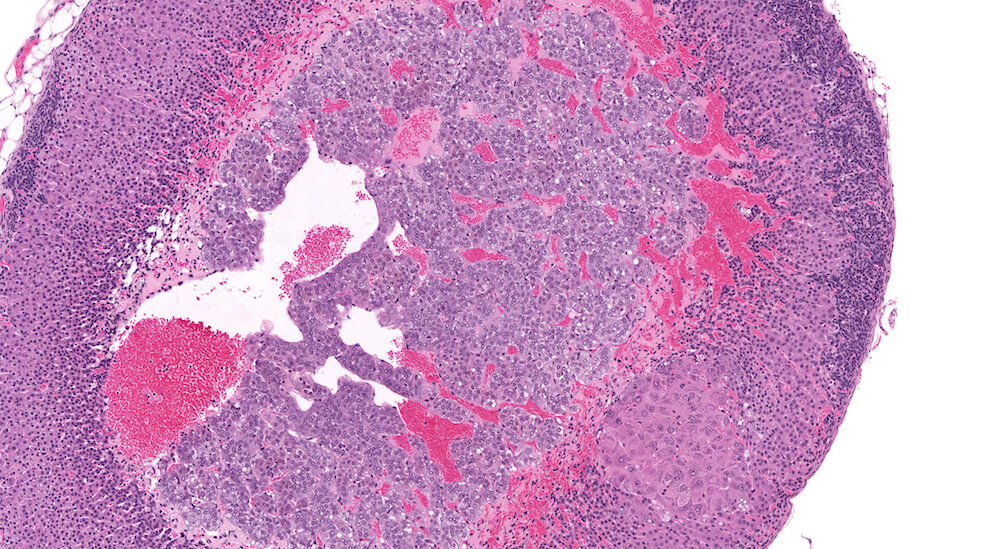
Figures 30a (A81967) & 30b (A81968). Subcapsular hyperplasia: Treated female B6C3F1 mouse in a 2-year toxicity/carcinogenicity study. As is typical in chronic toxicity/carcinogenicity studies, there are multiple changes in this adrenal. There is a mild to moderate band of subcapsular hyperplasia and a small focus of cortical hypertrophy in the zona fasciculata (Figures 30a & 30b). The x-zone has undergone nearly complete involution with a band of congestion remaining at the corticomedullary junction. Diagnosis of subcapsular hyperplasia and focal cortical hypertrophy are recommended for this adrenal while a diagnosis of x-zone involution would be dependent upon comparison to the degree of x-zone involution in concurrent study controls.
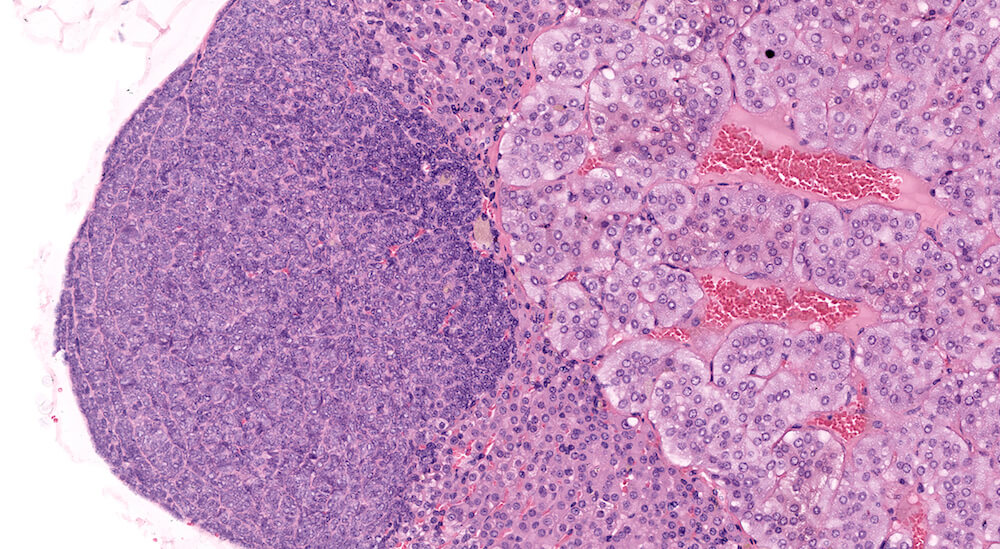
Figures 31a (A81856) & 31b (A81857). Focal subcapsular hyperplasia: Vehicle control male B6C3F1 mouse in a chronic study. This very discrete proliferation of basophilic subcapsular cells is present without significant compression of adjacent parenchyma but has protruded outward changing the normal configuration of the adrenal (Figures 31a & 31b). Subcapsular hyperplasia is common in several strains of mice, may be multifocal, increases with age, and can progress to subcapsular adenoma. This example is a borderline subcapsular adenoma but was diagnosed as hyperplasia due to absence of compression.
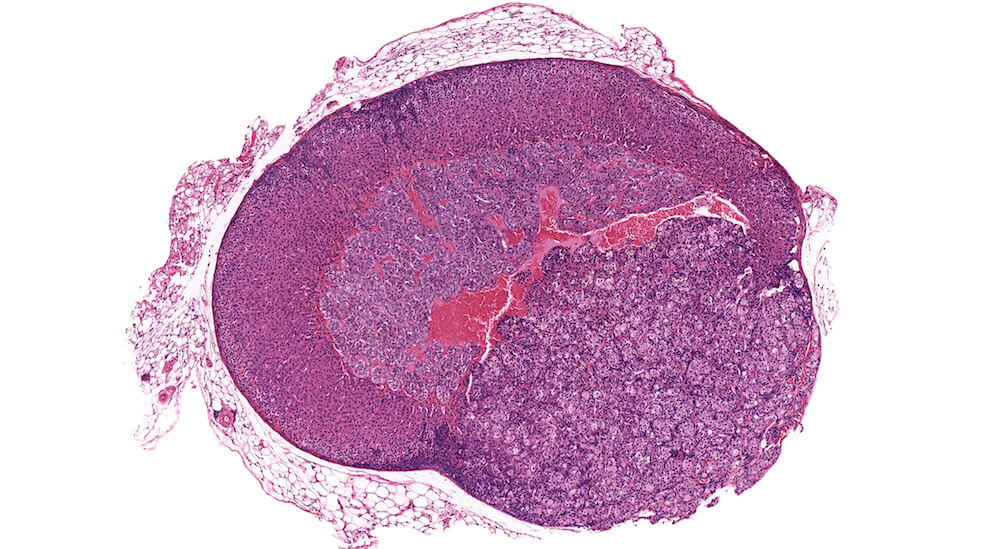
Figure 32 (A81840). Subcapsular adenoma: Vehicle control male B6C3F1 in a chronic toxicity/carcinogenicity study. In this example, a diagnosis of subcapsular adenoma is based on compression of the adrenal medulla and excessive protrusion above the normal contour of the adrenal surface. The adenoma is comprised chiefly of Type Bsubcapsular cells with smaller amounts of Type A spindeloid cells. The uneven protruding edge is a result of tissue handling; this neoplastic growth has not protruded through the adrenal capsule. A small area of focal subcapsular hyperplasia is present beneath the adrenal capsule on the side of the adrenal opposite the adenoma.
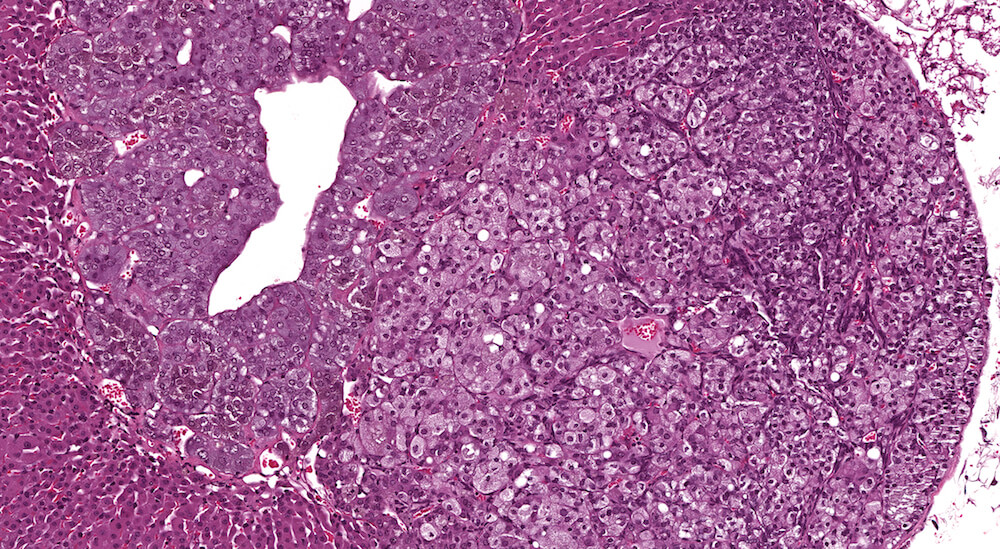
Figures 33a (A81843) & 33b (A81844). Subcapsular adenoma: High dose male B6C3F1 in a two-year toxicity/carcinogenicity study. This protruding proliferative mass has altered the normal adrenal contour and is compressing the cortex and medulla (Figure 33a). This neoplasm is comprised primarily of Type B polygonal cells with finely stippled pale eosinophilic cytoplasm along with a small component of Type A fusiform cells. In this case, diagnosis of adenoma is based on the degree of compression although it is recognized that the distinction between subcapsular hyperplasia and subcapsular adenoma is often unclear and diagnostically challenging.

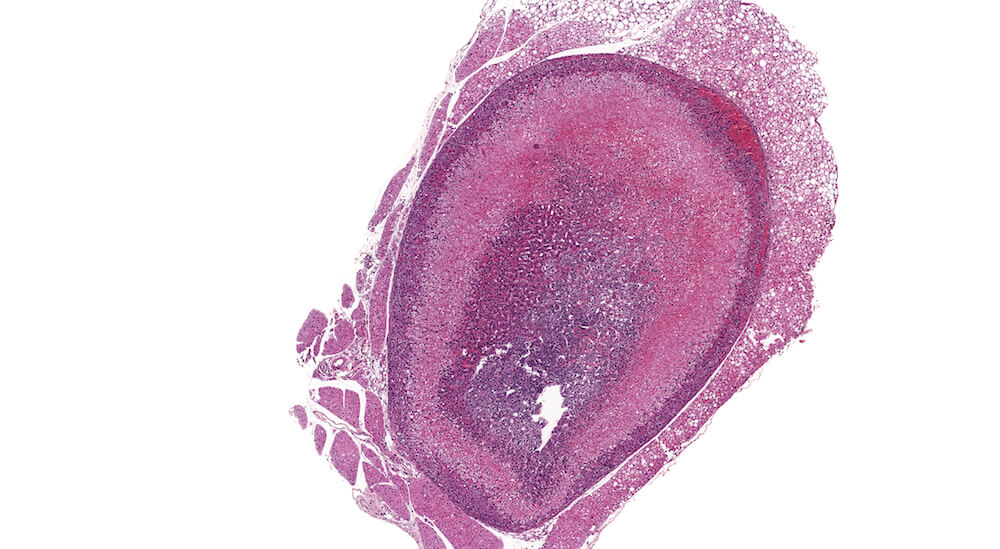
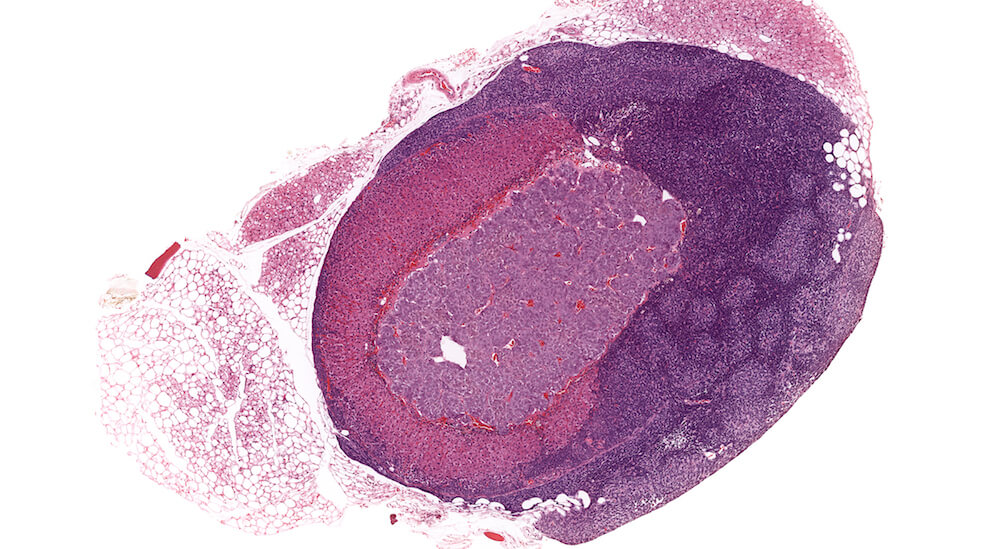
Figures 34a (A81849), 34b (A81851) & 34c (A81853). Subcapsular carcinoma: High dose female B6C3F1 mouse in a chronic study. A deeply basophilic cellular growth occupies approximately half of the adrenal cortex (Figure 34a) and has infiltrated through the capsule and into adjacent periadrenal adipose tissue (Figures 34b & 34c). The proliferative tumor is comprised of tightly packed basophilic cells with minimal visible cytoplasm (Figure 34c) interspersed with focal areas where tumor cells stain lighter due to moderate amounts of eosinophilic cytoplasm (Figures 34a & 34b). Subcapsular carcinomas are rare. Diagnosis is based primarily on cytological features, local invasiveness, and extension through the adrenal capsule. The X-zone is almost completely involuted in this adrenal.
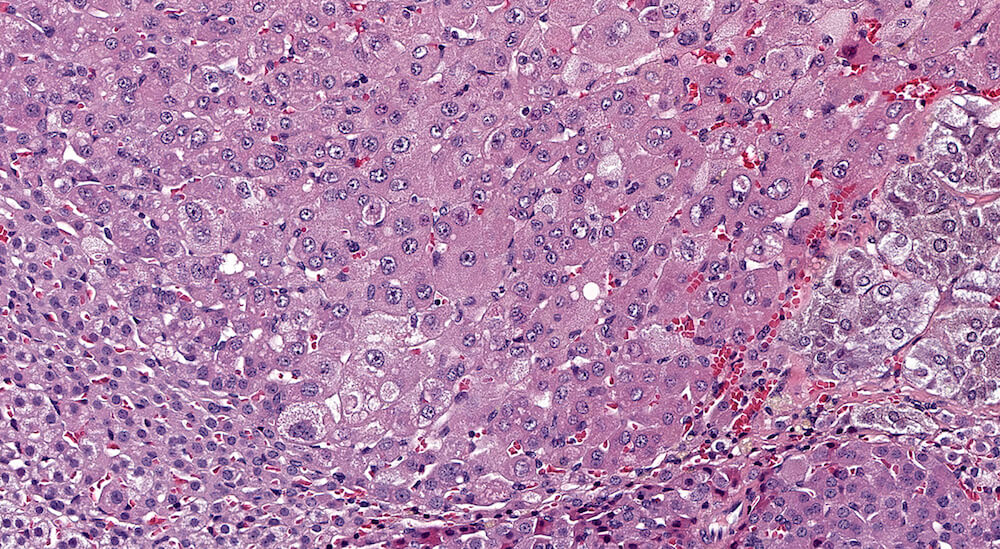
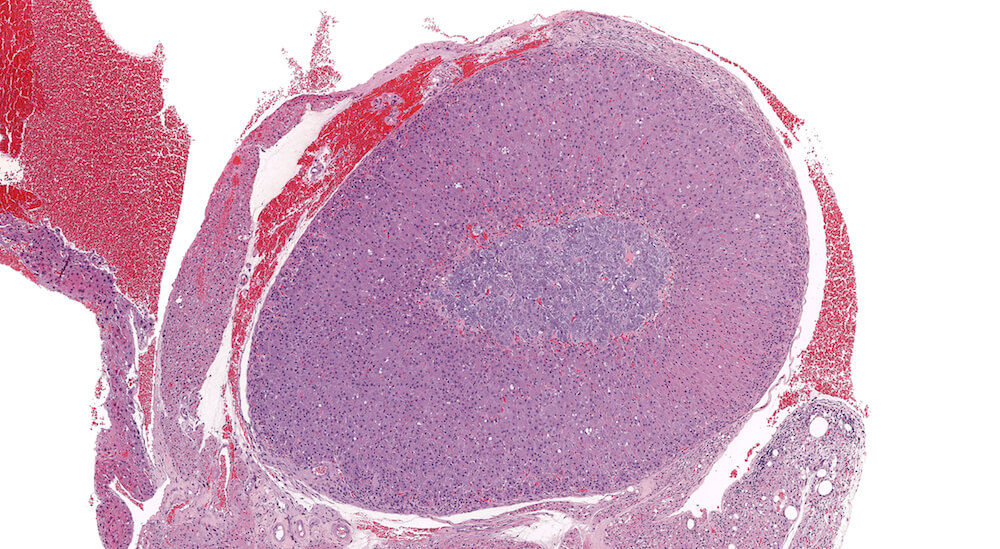
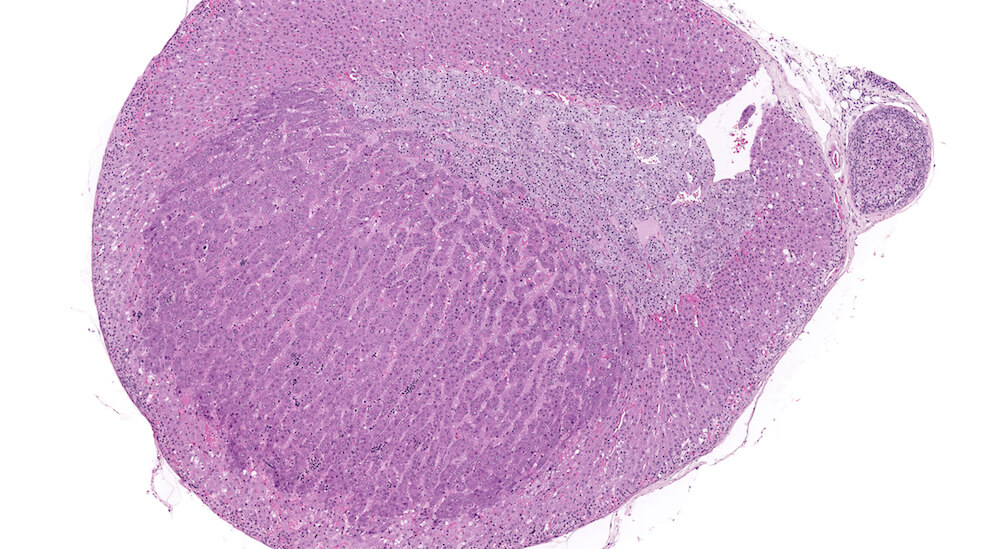
Figures 35a (A81868), 35b (A81869), & 35c (A81871). Cortical adenoma: Treated male B6C3F1 in a chronic study. An expansile growth consisting of enlarged eosinophilic cells is present in the zona fasciculata and has caused compression and distortion of the cortex and medulla. Cells comprising the adenoma are enlarged in contrast to the normal zona fasciculata cells, lack the normal organizational structure of the zona fasciculata, and vary in cell and nuclear size. The diagnosis of adenoma is based on cytological features and the presence of compression of adjacent more normal adrenal parenchyma. A focal area of cortical hyperplasia is present in the zona fasciculata adjacent to the adenoma (Figure 35b), is comprised of enlarged amphophilic cells, and merges with the adjacent parenchyma without causing compression. A small focus of subcapsular hyperplasia is present near one pole of the adrenal and an accessory cortical nodule is present in the adjacent periadrenal fat near one pole of the adrenal (Figure 35a).
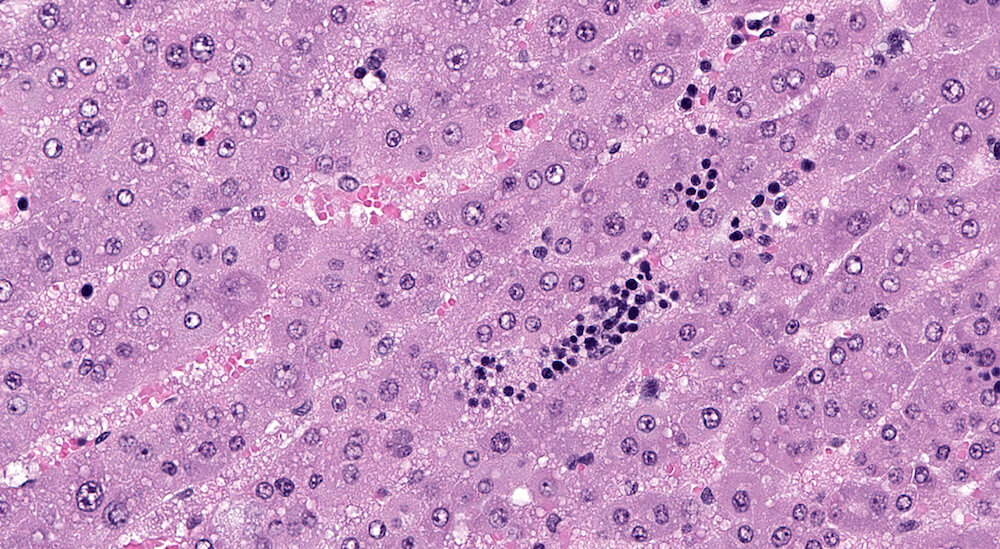
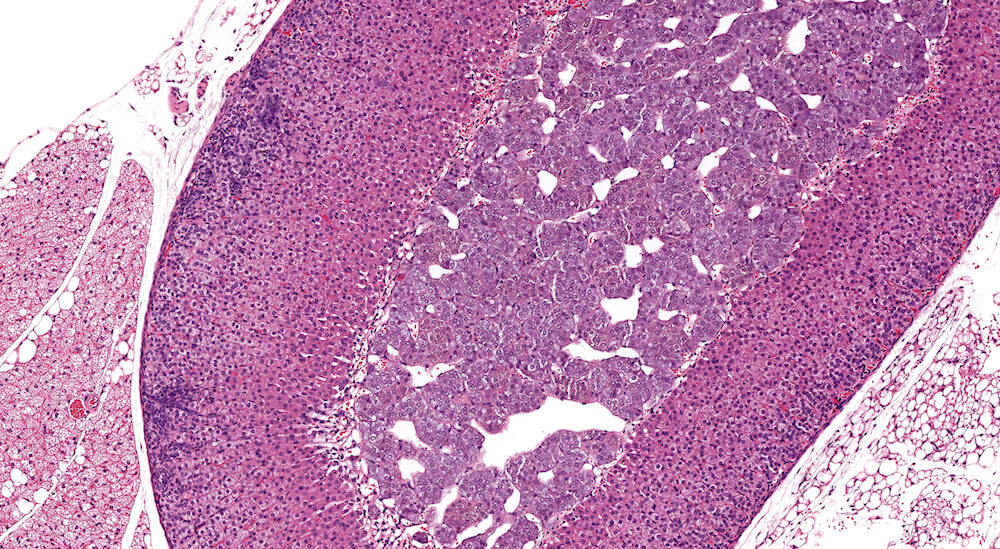
Figures 36a (A81862), 36b (A81863), 36c (A81864), & 36d (A81865). Cortical adenoma: Vehicle control male B6C3F1 mouse in a chronic study. An expansile growth comprised of tinctorially distinct cells that are larger than adjacent zona fasciculata cells are arranged in small nests and trabeculae with compression of the medulla (Figures 36a. 36b, & 36c). The diagnosis of cortical adenoma is based on the cytological features, abnormal growth pattern, and compression of the medulla. Extramedullary hematopoiesis is present with in the adenoma (Figures 36b & 36d). An accessory cortical nodule is present near the hilus (Figure 36a).
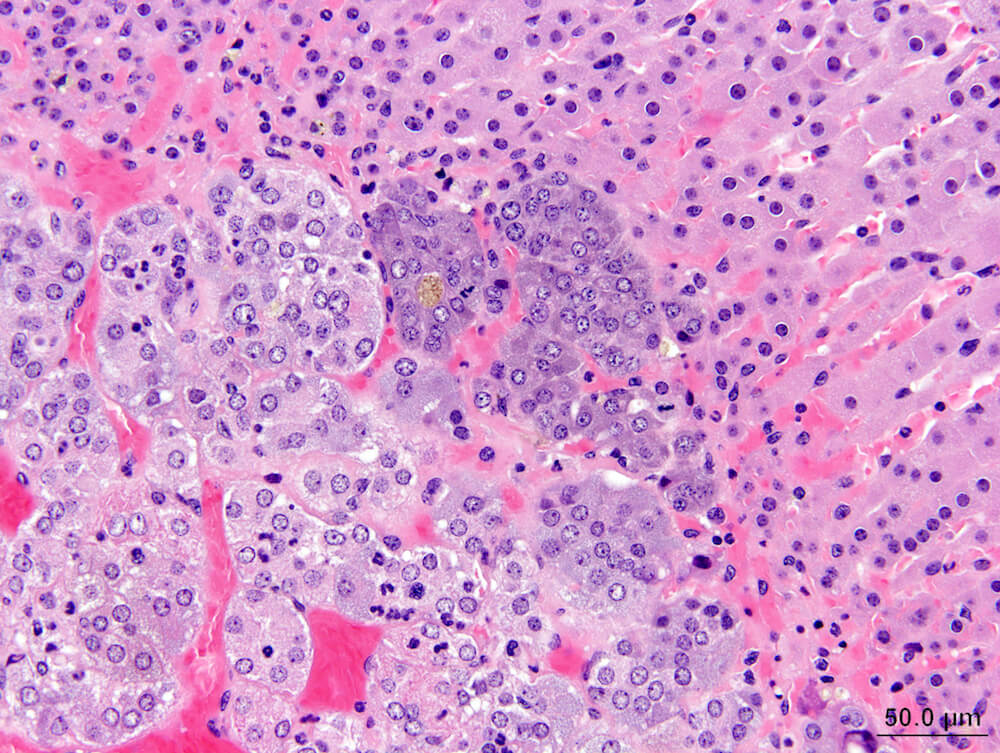
Figures 37a (A56654) & 37b (A56653). Focal medullary hyperplasia: Treated male B6C3F1 in a 2-year study. Focal clusters of basophilic medullary cells present at the corticomedullary junction are comprised of closely packed cells in contrast to the more normal medullary parenchyma (Figures 37a & 37b). There are occasional mitotic figures in the focal hyperplastic clusters. Pyknotic nuclear fragments consistent with apoptosis are present in the medulla adjacent to the focal hyperplasia (Figure 37b).
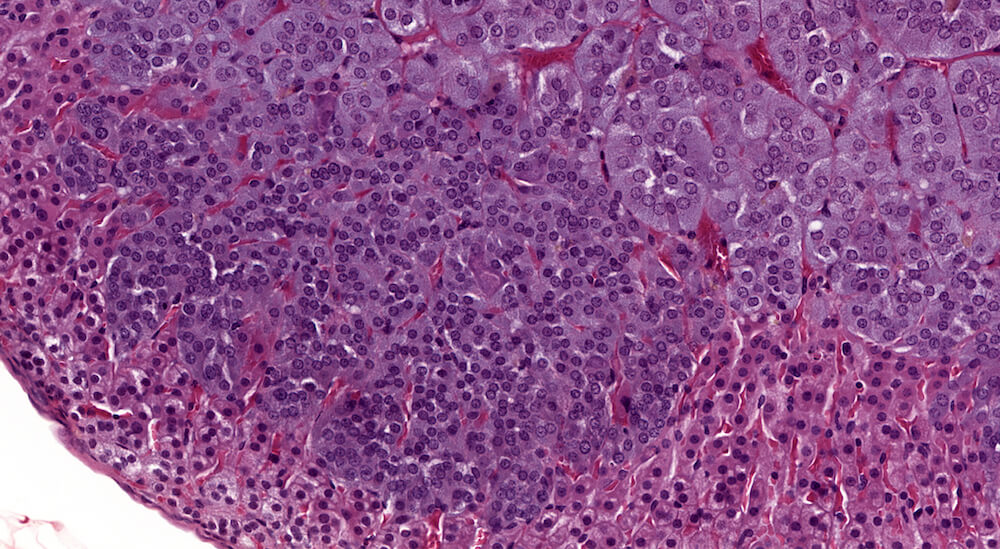
Figures 38a (A81959), 38b (A81961), & 38c (A81962). Diffuse medullary hyperplasia: Treated male B6C3F1 mouse in a 2-year study. Medullary hyperplasia is usually focal in mice but may occasionally be diffuse involving up to 50% of medullary size. The adrenal medulla is distorted by irregular patches of hyperbasophilic cells extending into the adrenal cortex (Figures 38a & 38b). The cells are arranged in small nests of darkly stained compact cells extending into the zona fasciculata (Figure 38b). In some areas, less clearly delineated clusters of tightly packed cells are present within the zona fasciculata (Figure 38c). Despite the extensive and multifocal proliferation of medulla cells, there is little evidence of compression of adrenal cortex in this enlarged adrenal gland.
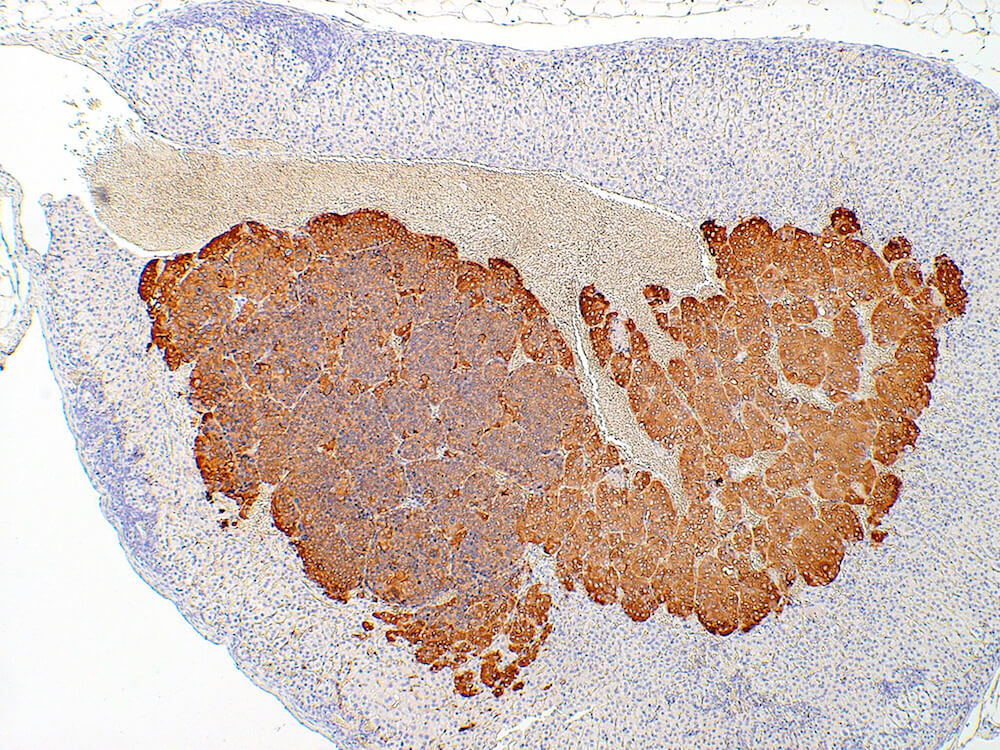
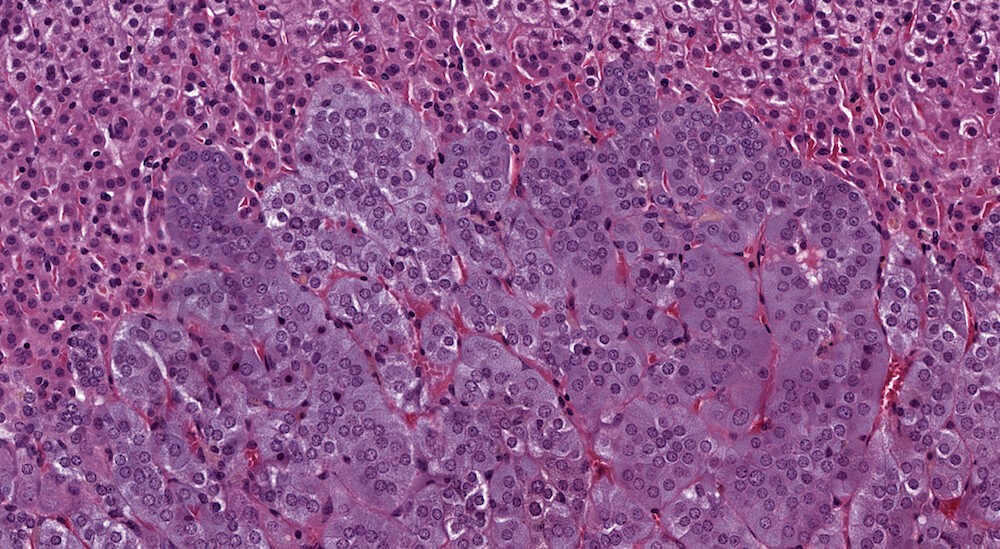
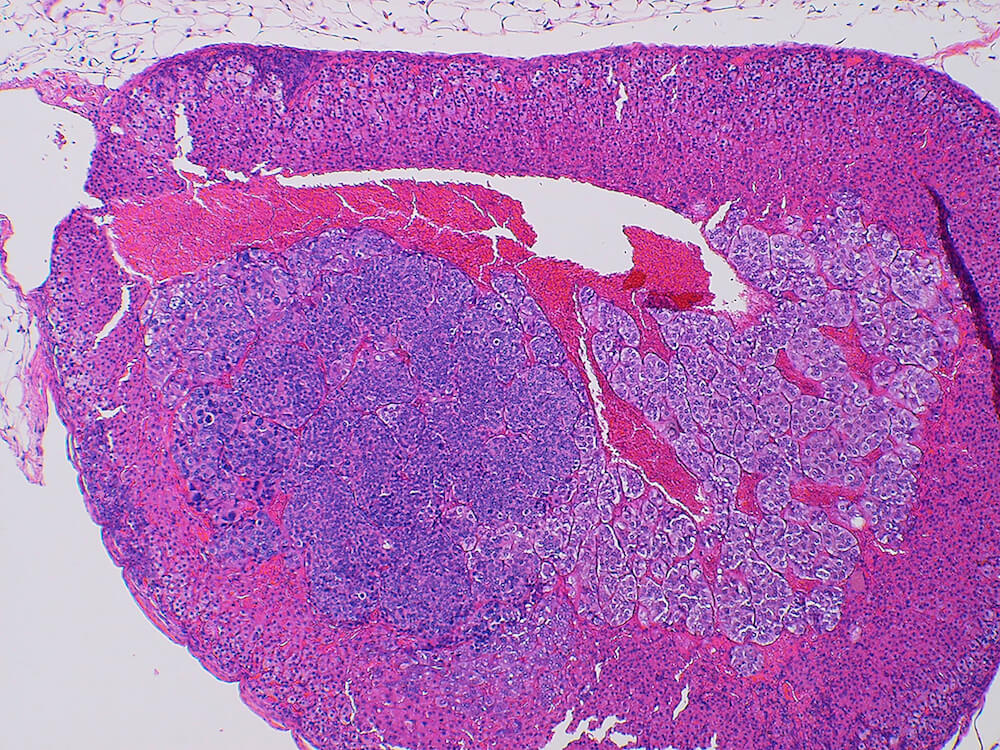
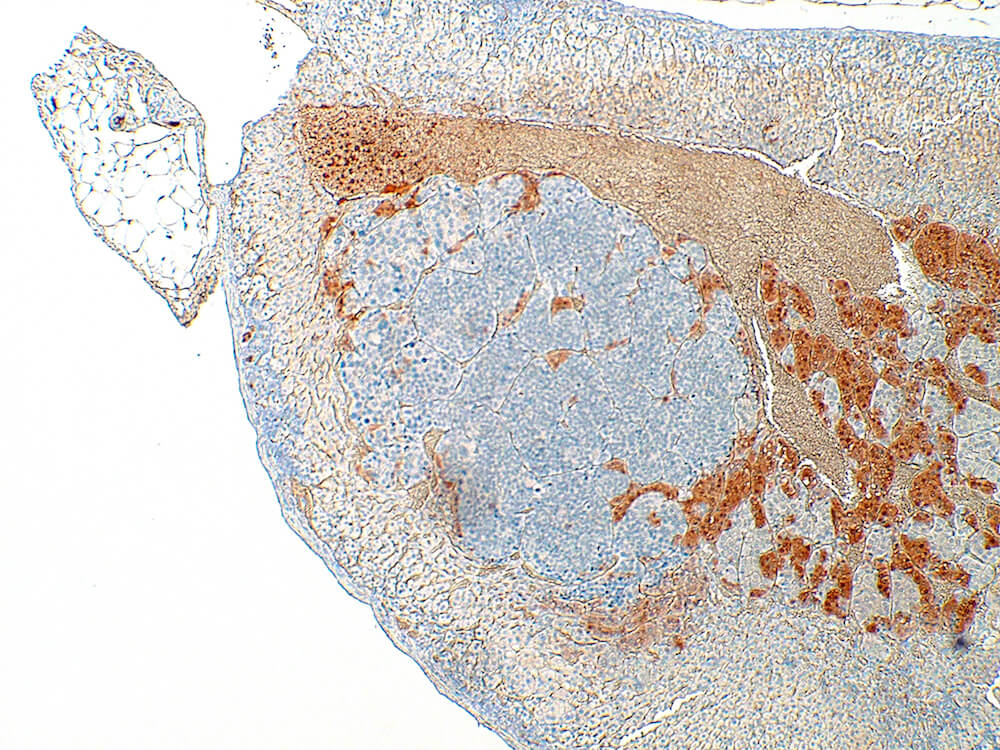
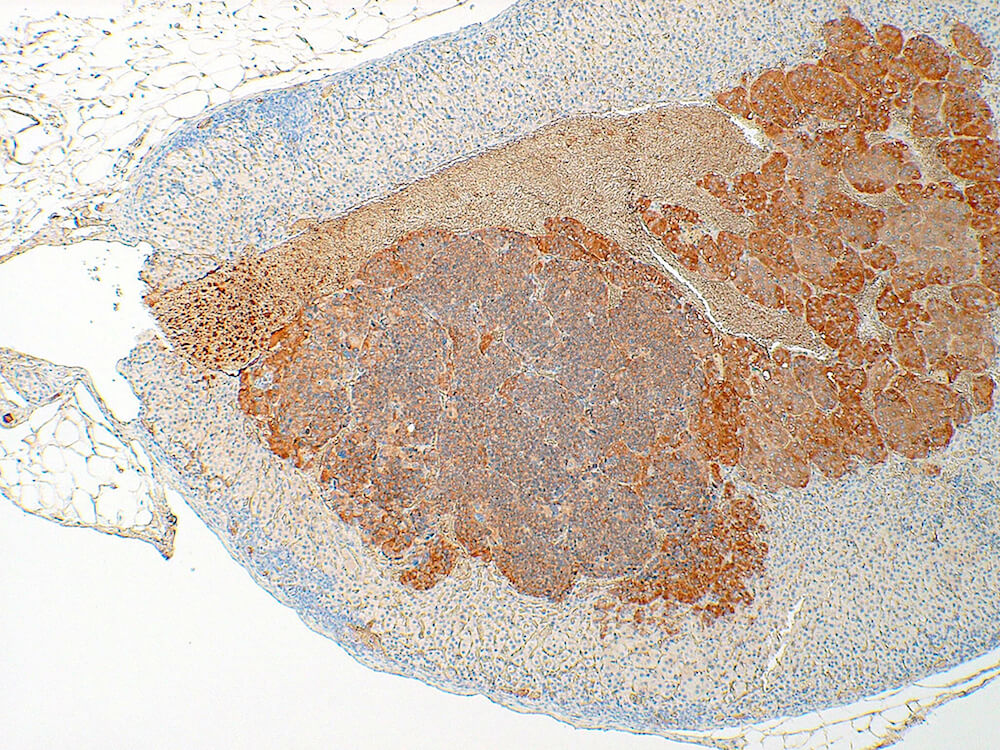
Figure 39a (H&E), 39b (PNMT IHC), 39c (CGA IHC) and 39d (TH IHC). Pheochromocytoma: Control male B6C3F1 in a chronic study. Irregular nests of a deeply basophilic pheochromocytoma has extended into the cortex and is mildly compressing the normal medulla (Figure 39a). In immunohistochemical staining for phenylethanolamine -N- methyltransferase (PNMT), a rate limiting enzyme in catecholamine synthesis, the normal medulla is strongly positive while the pheochromocytoma is largely negative except for occasional scattered immunopositive cells (Figure 39b). Both the normal medulla and the pheochromocytoma are immunopositive for chromogranin A (CGA) with more intense staining in the normal medulla (Figure 39c). CGA is a component of secretory granules present in chromaffin cells. Both normal medulla and pheochromocytoma are strongly immunopositive for tyrosine hydroxylase (TH), a rate limiting enzyme in catecholamine synthesis. A minimal amount of subcapsular hyperplasia is present in this adrenal gland.
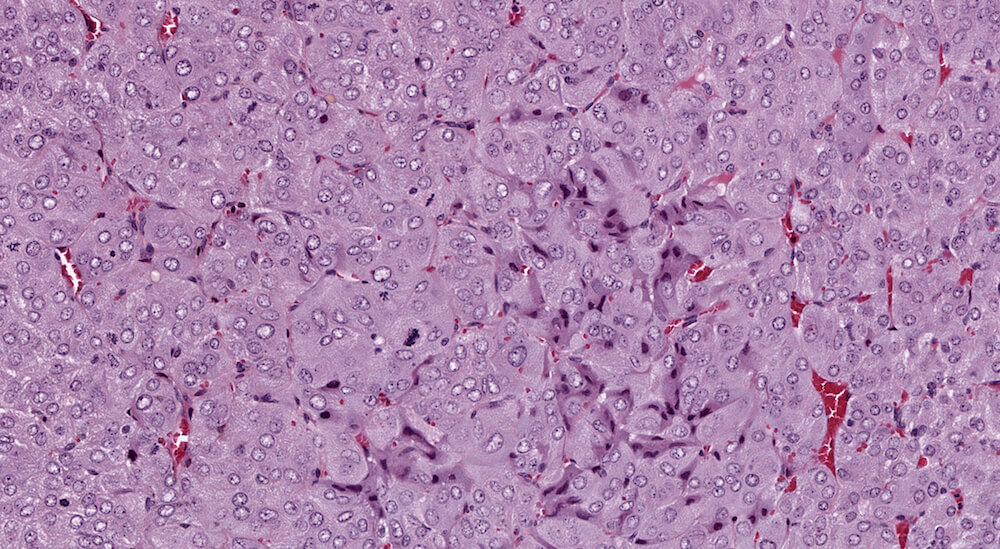
Figure 40a (A81963), 40b (A81964), 40c (A81965) and 40d (A81966). Pheochromocytoma: Treated male B6C3F1 mouse in a chronic study. The adrenal medulla is markedly enlarged by tightly backed nests of cells with compression of the adrenal cortex (Figures 40a & 40b). A few tumors cells within the tumor have angular shapes, darker staining cytoplasm, and pyknotic nuclei indicative of degeneration (Figures 40c & 40d). Numerous mitotic figures are present in this tumor (Figure 40d).
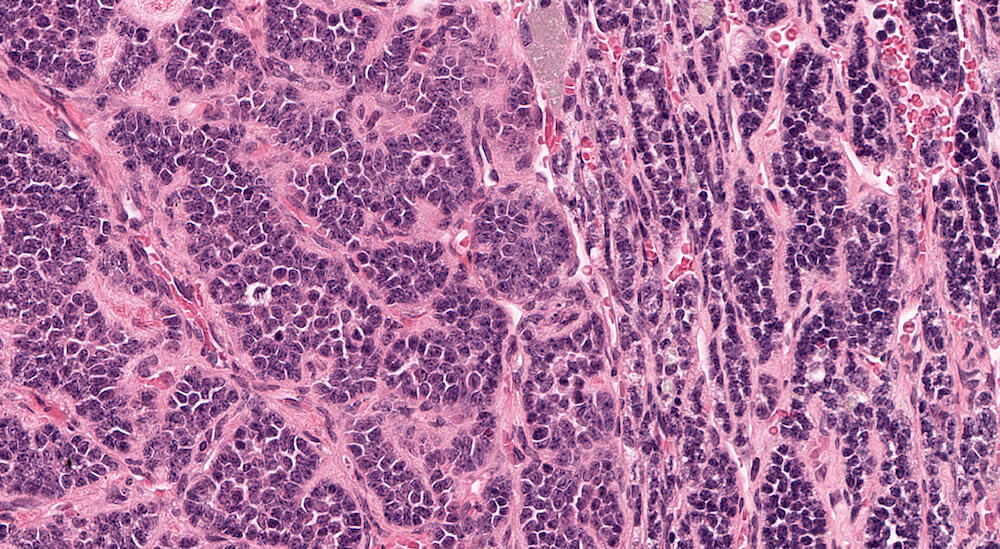
Figure 41a (A81977), 41b (A81979), 41c (A81951) and 41d (A81952). Neuroblastoma: Untreated 156-week old B6C3F1 mouse in an aging study. The adrenal has been effaced by multiple irregular lobes of densely cellular growth comprised of irregular nests and ribbons of tightly packed round basophilic nuclei separated by bands of finely fibrillary eosinophilic material consistent with neuroblastoma. The neoplastic cells have minimal amounts of cytoplasm. The eosinophilic material separating the neuroblastoma cells represents neurofibrillary fibers.