ACKNOWLEDGEMENT AND DISCLAIMER: This is a draft chapter that has not been peer reviewed. While its production and figures were supported by the National Toxicology Program/National Institute of Environmental Health Sciences, it solely represents the opinion of the author, R. R. Maronpot.
••••••••••••••••••••••••••••••••••••
Introduction
The pituitary is functionally a master endocrine tissue involved in homeostasis and regulation of growth, reproduction, sexual maturation, pregnancy, metabolism, lactation, and stress responses. The adenohypophysis includes the anterior and intermediate lobes. Cellular components of anterior lobe produce growth hormone, prolactin, thyroid stimulating hormone, leutotrophic hormone, and follicle stimulating hormone that function as trophic factors for peripheral target tissues. The neurohypophysis (posterior pituitary) consisting of the pars nervosa and infundibular stalk produces oxytocin and vasopressin (antidiuretic hormone). The two parts of the pituitary are functionally controlled by the hypothalamus (hypothalamic-pituitary axis) and feedback mechanisms from hormonal target tissues. Studies during the past decade using genetically modified mice have provided significant understanding of human pituitary dysfunction and provided valuable insight into the importance of pituitary progenitor/stem cells and the plasticity of pituitary responses in maintaining homeostasis.
Embryology
The mouse pituitary gland (hypophysis) consists of an anterior and posterior lobe. As with other endocrine organs, there is separate embryological origin of the two components. The anterior pituitary (adenohypophysis) originates from Rathke’s pouch (an oropharyngeal diverticulum) while the posterior pituitary (neurohypophysis) forms from neuroectoderm on the floor of the 3rd ventricle (diencephalon) (Kerr 1946; Kaufman 1994; Kaufman et al., 2010). In the mouse, the placode that forms Rathke’s pouch (RP) is present at 7.5 days post coitum (dpc) with formation of a rudimentary RP at approximately 8.5 to 9 dpc (Kelberman et al., 2009; Dubois & El Amraoui 1995). A day later an infundibulum recess develops from neuroectoderm on the floor of the 3rd ventricle (ventral diencephalon) to ultimately become the posterior pituitary and pituitary stalk. This neuroectoderm forms in juxtaposition with RP.
The juxtaposition of the neural and oral ectoderm is maintained throughout subsequent development of the pituitary and allows for inductive interaction during development and formation of the anterior and posterior pituitary (Takuma et al., 1998; Kelberman et al., 2007). Multiple transcription factors and genes are involved in the highly coordinated sequential steps in cellular commitment and positioning of pituitary cell lineages, all responsive to intrinsic and extrinsic signaling gradients (Cushman et al. 2001; Dasen & Rodenfeld 2001; Dasen & Rosenfeld 1999; Asa & Ezzat 1999; Kelberman et al. 2009; Rizzoti 2015). Corticotrope, thyrotrope, somatotrope, lactotrope and finally gonadotrope cells develop in association with specific gene expression between 12.5 and 16.5 dpc (Kelberman et al., 2009). Starting at about 14 dpc, the internal carotid artery provides branches to the hypothalamic area to ultimately become the hypophyseal portal system, the main blood supply to the anterior pituitary (Kaufman et al., 2010). The hypophysis and pituitary stalk receive blood from superior hypophyseal arteries. Starting at about 15 dpc, progressive rapid proliferation of anterior and posterior pituitary and the vascular supply commences, along with obliteration of the lumens of PR and posterior pituitary and early ossification of the sella turcica. Published histologic features showing progressive pituitary gland development up to approximately 18 dpc are clearly shown in stained sections (Kaufman et al., 2010). At this stage of development there is merging of anterior and posterior pituitary with compression of RP and further maturation of the pituitary gland. Ultrastructural demonstration of granular cells producing thyroid stimulating hormone (TSH), follicle stimulating hormone (FSH), leutinizing hormone (LH), and somatotrophic hormone (STH) is present at gestation day 16 in SMA mice following by a proportional increase in STH producing cells (Sano & Sasaki 1969). Throughout the process of pituitary organogenesis there is active migration of progenitor cells, cell patterning and cell differentiation under the influence of multiple growth and transcription factors (Zhu et al., 2007; Dasen et al., 1999; Dasen et al., 2001; Rizzoti & Lovell-Badge 2005). After birth, there is continued proliferation and differentiation of hormone-producing cells as well as establishment of stem/progenitor cells (Levy 2002; Levy 2008; Nolan et al., 1998).
Anatomy
The mouse pituitary is located in a midline shallow depression, the sella turcica, on the dorsal surface of the basiphenoid skull bone. A dual tissue origin results in an anterior (adenohypophysis) and posterior (neurohypophysis) pituitary gland that fuse during embryogenesis with the point of fusion becoming the pars intermedia. The adenohypophysis represents the major portion of the pituitary gland and consists of the pars distalis (pars anterior), the pars intermedia, and the pars tuberalis. A cleft remnant of Rathke’s pouch is located between the pars distalis and pars intermedia. The neurohypophysis (pars posterior) includes the median eminence of the tuber cinereum, infundibular stalk, and pars nervosa. The neurohypophysis connects to the hypothalamus by the infundibular stalk. The small pars tuberalis partially wraps around the infundibular stalk.
When the brain is removed at necropsy, the pituitary stalk attachment to the brain is severed. Since the mouse pituitary is small and fragile, it is often fixed in situ for subsequent histologic examination. The histological preparation is typically a coronal section showing the pars distalis, pars intermedia and pars nervosa without the infundibular stalk and pars tuberalis. The pituitary is larger and heavier in females with weight differences among mouse strains (Liebelt 1994).
Histology
Adenohypophysis
The bilobed pars distalis, the largest component of the adenohypophysis, surrounds the pars intermedia and pars nervosa and is comprised of nests and cords of round to polygonal cells interspersed within a rich fibrovascular plexus. In hematoxylin and eosin-stained sections, pars distalis cells are chromophilic being tinctorially acidophilic or basophilic or alternatively are chromophobic (Figures 1 & 2). Chromophilic cells contain secretory cytoplasmic granules that account for their tintorial staining. Acidophilic chromophils are characterized by small round or oval cells with a central nucleus while basophilic chromophils are more polyhedral with an eccentric nucleus (Leibert 1994). Chromophobic cells have a large nucleus with 1 or 2 nucleoli, lack obvious secretory granules, and may serve as resting reserve cells or simply be in an early active synthesis phase. Immunohistochemistry has been used to more definitively identify specific cell types in the adenohypophysis (Sasaki & Iwama 1988; Sano & Sasaki 1969; Capen et al., 1991; Liebelt 1994). Several distinctive cell types of the mouse adenohypophysis have been identified by electron microscopy (Liebelt 1994). Histomorphology as well as ultrastructural cytologic features may be influenced by sex and the physiological state of the pituitary. Sex differences in the proportions of lactotrophs and somatotrophs have been documented by immunohistochemistry and electron microscopy in SMA mice along with documentation of presence of a few mammosomatotrophs (Sasaki & Iwama 1988).
The proportion of each cell type varies dynamically depending upon physiological conditions. Pars distalis cells are responsible for secretion of major pituitary hormones and include somatotrophs that secrete growth hormone and mammotrophs that secrete prolactin in females. Somatotrophs and mammotrophs are typically acidophilic in routine H&E stains. Basophilic gonadotrophs produce follicle stimulating hormone and leutinizing hormone, are more numerous in males than in females, but their frequency in females varies depending upon the estrus cycle stage. Thyrotrophs are also basophilic and secret thyroid stimulating hormone. However, tinctorial features of pituitary cells, especially in the pars distalis, may not accurately reflect phenotype and cell type frequency. Using immunohistochemistry, Baker and Gross concluded that the frequency and distribution of hormone producing cells in the pars distalis of Swiss-Webster mice were similar to that in other species (Baker & Gross 1978).
The adult rodent pituitary has a low steady-state cell turnover under both hypothalamic neuroendocrine control and feedback control from peripheral target tissues (Fauquier et al. 2008; Nolan et al. 1998). Dynamically changing pituitary hormonal responses to changing physiology are controlled by a complex constellation of molecular transcription factors and genes during development and in adulthood as well as during pituitary neoplasia (Zhu et al. 2007; Asa & Ezzat 1999; Sav 2014). The original notion that different chromophilic pituitary cells were each specialized to only produce a specific hormone is no longer valid. Pituitary cells are plurihormonal and proportions of trophic cells in mice vary under differing conditions as a reflection of pituitary plasticity. Examples include increases in lactotrophs in nursing mice, strain difference in high and low incidence mammary tumor strains (Sasaki et al. 1978); increased gonadotrophs following castration in mice (Levy 2008; Inoue & Kurosumi 1981); and increase in corticotrophs in cold stressed male and female mice (Senovilla et al. 2008). We now better appreciate the importance of pituitary flexibility in adjusting its hormone producing repertoire in response to changing physiological dynamics, including pregnancy, lactation, metabolism, stress, and age.
The plasticity of pituitary hormonal response comes from recruitment of uncommitted chromophobe cells (Levy 2002), transdifferentiation of committed cells (Nunez et al. 2003; Vidal et al. 2001), and/or cell division (Levy 2002) but for rapid responses to changing physiological conditions, cell proliferation is not likely to be a major contributor (Nolan et al 1998; Levy 2008). Also contributing to pituitary plasticity in response is recruitment of cells from the stem/progenitor cell compartment (Vankelecom 2010; Rizzoti et al. 2013). It is estimated that 1.5% of mouse anterior pituitary cells are multipotent progenitor/stem cells (Florio 2011). SOX2-expressing progenitor cells responding to hormonal demands are capable of generating all major cells types in the adult mouse pituitary. SOX2+/SOX9+ identifies transit-amplifying cells and SOX2+/SOX9- cells are multipotent progenitor/stem cells (Fauquier et al. 2008; Florio 2011).
Among the chromophobic cells of the pars distalis is a small population of non-secretory folliculo-stellate cells that have properties of dendritic cells and are variably immunoreactivity for S-100 protein. Their phenotype suggests a lymphoid/mesenchymal anlage in pituitary development in addition to the known neuroectoderm and oral ectoderm tissue anlage (Allaerts et al. 1991; Allaerts et al. 1993; Vankelecom et al. 1993).
Cells of the pars intermedia are larger than those of the pars distalis, are polygonal with densely stained nuclei, and are arranged in groups separated by connective tissue and vascular channels (Liebelt 1994)(Figures 1 & 2). Using fluorescent immunohistochemistry, a-melanocyte stimulating hormone (MSH), b-MSH and adrenocorticotrophic hormone (ACTH) producing cells have been identified in the pars intermedia using fluorescent immunohistochemistry with enhanced excretion by these cells after dehydration and adrenalectomy (Roux & Dubois 1976). The pars intermedia and pars nervous are separated by a capillary plexus with contact zones where the pars intermedia and pars nervosa have direct communication (Jarskar 1977).
There is increasing evidence that a subpopulation of non-hormonal cells function as stem/progenitor cells in the embryonic and adult mouse pituitary gland. These stem cells express high-mobility group (HMG) SOX2 and SOX9 transcription factors and are located around the pituitary cleft (remnant of Rathke’s pouch) as well as scattered in the anterior lobe (Rizzoti 2015; Rizzoti et al., 2013; Andoniadou et al., 2013; Vekelecom 2010; Fauquier et al., 2008). They actively migrate during embryonic and postnatal pituitary organogenesis and in the adult mouse reside in niches where they maintain their stemness (Yoshida et al., 2016).
Neurohypophysis
The neurohypophysis consists of the pars nervosa, the infundibular stalk, and the median eminence of the tuber cinerum of the hypothalamus. The neurohypophysis is hypocellular and mostly occupied by unmyelinated axons of neurosecretory magnocellular neurons originating in the supraoptic and paraventricular nuclei of the hypothalamus (Kelberman et al., 2009) (Figures 1 & 2). The axons terminate in association with a rich capillary network that is supported by modified glial cells (pituicytes). Precursors for the hormones oxytocin and vasopressin are synthesized in the hypothalamic supraoptic and paraventricular nuclei, transported along the axons, stored in axon terminals for subsequent cleavage into active hormone with ultimate release into the vascular capillary network.
Vascular supply
Capillary networks develop in the mouse embryo pituitary as early as gestation day 16 and continue to extend into the parenchyma throughout embryogenesis (Hashimoto et al., 1998). Blood from branches of the internal carotid arteries and the circle of Willis form narrow capillaries in the posterior lobe with the pars intermedia being relatively avascular. A rich blood supply penetrates the pars tuberalis that provides a scaffold for a capillary plexus starting at the median eminence with ultimate drainage into the hypophyseal portal plexus that supplies the pars distalis (Kaufman et al., 2010; Capen 1996).
Physiology
Sometimes referred to as the master gland, the pituitary is controlled by the neurosecretory neurons in the hypothalamus that produce small peptides called releasing hormones and releasing-inhibitory hormones that enter the hypophyseal portal system to act on specific cell types in the adenohypophysis. The releasing hormones bind to specific receptors to initiate Ca2+ signals and subsequent secretion of the appropriate trophic hormone that is specific for a given target tissue. The whole endocrine system is kept in balance by a feedback mechanism whereby the blood concentration of a specific target tissue hormone acts on the hypothalamus to up- or down-regulate production of releasing or releasing-inhibitory signals from the hypothalamus. As examples, TSH is inhibited by T3/T4 from the thyroid; FSH and LH by androgens and estrogens; ACTH by adrenal corticosteroids; and growth hormone (GH), prolactin and MSH by their corresponding release inhibiting hormones.
Anterior Pituitary
Pars Distalis
The anterior pituitary secretes seven endocrine hormones controlled by releasing and inhibitory factors (Table 1): prolactin from mammotrophs, luteinizing hormone and follicle-stimulating hormone from gonadotrophs, growth hormone from somatotrophs, thyroid stimulating hormone from thyrotrophs, adrenocorticotrophic hormone from corticotrophs, and melanocyte-stimulating hormone from melanotrophs. The proportion of the various hormone producing trophic cells fluctuates as a dynamic reflection of changing physiology (Levy 2002). For example, during lactation numerous acidophils and chromophobe cells may be produced with the acidophils generated primarily from transition of chromophobe cells and to a much smaller extent from cell division. Other types of cells show differences associated with sexual maturity (Yamada et al., 1957). Strain differences in number and size of prolactin secreting cells along with differences in ultrastructural features suggest that secretory activity in prolactin secreting cells is higher in diestrus female C3H than in C57BL mice (Sasaki et al. 1979). Cortisol injections in sexually mature C57BL/6J mice results in reduced numbers of mammotrophs and increased numbers of somatotrophs while adrenalectomy is associated with increases in mammotrophs and decreases in somatotroph numbers (Keough and Wood 1978). Pulsatile hormone release to control homeostasis in response to changing physiology and metabolism also likely involves modulation of blood flow (Schaeffer et al. 2010).
Table 1. Pars Distalis Cells and Their Controls.
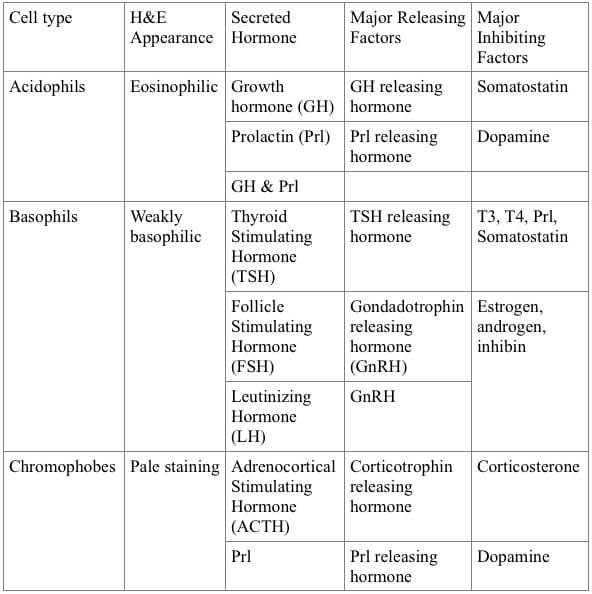
Folliculo-stellate cells located in the adenohypophysis form a functional network of intrapituitary communication. The narrow cytoplasmic processes of folliculo-stellate cells extend between glandular cells providing circuitry than can transmit action potential rises in cytoplasmic Ca2+ signals to relay information across different regions of the pituitary gland and thereby fine tune highly orchestrated pituitary function (Fauquier et al. 2001). Folliculo-stellate cells express S-100, share similarities with CNS glial cells, and are immunopositive for dendritic cell aminopeptidase (Allaerts et al. 1991). They produce bioactive interlukin-6 to regulate cell division and hormone synthesis, thereby further establishing cross-talk between the immune and neuroendocrine systems (Vankelecom et al. 1993). While leptin is expressed primarily in thyrotrophs, it is also expressed in folliculo-stellate cells and these cells also have an isoform of a leptin receptor suggesting a possible autocrine/paracrine loop in the pituitary (Jin et al. 2000).
Pars Intermedia
The pars intermedia contains polygonal cells with pale staining foamy cytoplasm and is separated from the pars distalis by a cleft, a remnant of Rathke’s pouch (Figures 1 & 2). The mouse pars intermedia is larger than in other species and is the source of adrenocorticotrophic hormone, melanocyte stimulating hormone and beta-endorphin (Oishi et al., 1992). ACTH-secreting corticotrophs represent the major cell type in the pars intermedia, although a few corticotrophs may also be present in the pars distalis. Following adrenalectomy in the mouse, the pars intermedia becomes hyperactive with greater excretion of MSH and ACTH-like polypeptides (Roux and Dubois 1976). The pars intermedia is in direct contact with the pars nervosa being separated only by a capillary plexus without a basement membrane (Eurenius and Jarskar 1975) allowing intimate contact between these two parts of the pituitary. Adrenergic nerve terminals in the pars intermedia have synaptic-like contact with pars distalis glandular cells suggesting there is some direct nervous control in addition to humoral trophic effects on the pars intermedia (Jarskar 1977). MSH, ACTH and a corticotropin-like peptide have been demonstrated in the pars intermedia (Jarskar 1977). Despite these features of the pars intermedia, its exact functional significance requires further clarification.
Pars Tuberalis
The pars tuberalis is a main target of melatonin with a high density of the MT1 major melatonin receptor and thereby acts as an important interface in transmitting photoperiod information to the endocrine system (von Gall et al. 2005; Unfried et al. 2009). The melatonin action on the MT1 receptor leads to rhythmic expression of the clock genes (mPERa, mPER2, mCRY1), thereby driving prolactin secretion from the pars distalis (Morgan and Williams 1996; Jilg et al. 2005).
Posterior Pituitary
The unmyelinated axons of magnocellular secretory neurons located in the paraventricular and supraoptic nuclei of the hypothalamus transport secretory material to their terminal ends. The secretory material accumulates as variably sized spherical bodies known as Herring bodies and consists of molecular precursor molecules and neurophysin carrier protein that when cleaved will release active oxytocin and vasopressin into the associated vascular capillary network. Release of oxytocin and vasopressin is modulated by pituicytes that surround the neurosecretory axons and axon terminals with their cellular processes extending between the secretory endings and the basal lamina of fenestrated capillaries. Hormone release is under osmotic and receptor stimulated control and results in retraction of pituicyte processes, permitting increased contact of axon endings and capillaries (Hatton 1988). Triggering of oxytocin and vasopressin release by magnocellular secretory neurons relies on integration of reproductive, cardiovascular and osmotic afferent signaling as well as on regulatory features intrinsic to the magnocellular neurons themselves (Brown 2016). Vasopressin maintains bodily fluid balance and blood pressure and has vasoconstrictive properties. Oxytocin is important for parturition and lactation but has a large repertoire of many other activities and also acts in a neuroendocrine fashion to control or modulate release of prolactin and ACTH and possibly also gonadotrophins (Samson and Schell 1995).
Non-proliferative Changes
Pituitary Cysts
Pituitary cysts are incidentally seen in chronic mouse studies and may occur in the pars distalis and intermedia and rarely in the pars nervosa (Blumentahl 1955; Mahler & Elwell 1999; Liebelt 1994; Morton & Tekeli 1997). A 10 to 20% incidence has been documented in one study (Watanabe 1991). They may be associated with Rathke’s cleft but can occur randomly. The epithelial lining of unilocular or multilocular cysts may be cuboidal or columnar with or without cilia or irregularly lined by resident parenchymal cells. Their epithelial lining distinguishes them from angiectasis or dilated vascular channels. They are distinguished from cytic degeneration in that in the latter case spaces are lined by degenerating secretory cells. In rare cases cysts may have a squamous epithelial lining, suggesting thyroglossal duct origin. Cysts are typically filled with homogeneous or finely granular eosinophilic proteinaceous material (Figures 3 & 4). While cysts are presumptive developmental changes representative of the craniopharyngeal Rathke’s pouch origin, the possibility of cysts developing in mature mice cannot be excluded. Except in very unusual situations, it is not usually necessary to subclassify cysts based on presumed site of origin.
Degenerative Changes and Cytological Alteration
Truly degenerative pituitary lesions are not common in mice. Ablation of pituitary hormone target tissues including adrenal, thyroid, and gonads results in a hypertrophic and proliferative response in pituitary corticotrophs, thyroidtrophs, and gonadotrophs, respectively. In the case of gonadectomy in Wistar rats, the resulting gonadotrophs become enlarged and vacuolated and are referred to as “castration cells” (Inoue and Kurosumi 1981) and a similar response would be expected in mice. The increased volume of castration cells in rats is associated with increased rough endoplasmic reticulum, dilated endoplasmic reticulum cisternae, and increased numbers of secretory granules. Castration cells in mice are typically less prominent and less vacuolated compared to these cells in rats (Ranadive and Karande 1970). Development of “exhaustion cells” and “castration cells” are responses to changes in systemic physiology and could be considered adaptive changes rather than degenerative changes (Mahler and Elwell 1999). Mouse strain differences in gonadotroph responses following ovariectomy were earlier and more pronounced in C3H than in ICRC and C57 mice (Ranadive and Karande 1970).
Cystic degeneration, also referred to as cystoid degeneration, of the pars distalis may be seen in aging control mice as well as following treatment of female C3H HeN (MMTV+) with natural or synthetic estrogens (Cameron & Sheldon 1996; Firth & Ward 1988; Liebelt 1994). Cystic degenerative lesions lack an epithelial lining but rather are lined by parenchymal pituitary cells, some of which may show degenerative changes. Cytic degenerative lesions contain eosinophilic material with or without cellular debris (Figures 5 & 6).
Inflammatory and Vascular Changes
Inflammation restricted to the mouse pituitary is uncommon but may be seen as part of a more generalized process such as part of meningitis. Treatment induced hypophysitis and associated hemorrhage and atrophy is reported in SLJ/J female mice immunized with complete Freund’s adjuvant (Lupi et al. 2011). Following immunization with mouse pituitary extract, severe lymphocytic autoimmune hypophysitis with multinucleated cells was produced in SJL (Tzou et al. 2008). The pituitary gland is highly vascular and small areas of angiectasis may be seen as a common background change and are usually not diagnosed if of minimal severity.
Hypertrophic Lesions
Hypertrophy of the pars distalis and pars intermedia is usually a negative feedback response secondary to decreased function of target endocrine tissues. Hypertrophy may occur in combination with hyperplasia. Pars distalis hypertrophy is usually bilateral, may be generalized or occurring as multiple small clusters, and when extensive will significantly increase the size of the pituitary. The hypertrophic cells are readily apparent by increased volume of pale staining cytoplasm (Figures 7 & 8). Focal hypertrophy of the pars distalis can occur but is less common (Figures 9 & 10). Hypertrophy of the pars intermedia (Figures 11 & 12) is uncommon but likely generalized with the enlarged cells having increased cytoplasm volume, paler than normal staining, but retaining their structural arrangement in small groups and without an increase in nuclear density.
Proliferative Lesions
In general, pituitary tumorigenesis can occur via intrinsic pituitary abnormalities such as specific somatic mutations or via an abnormal regulatory mechanism such as escape from hypothalamic inhibition (Levy & Lightman 1993). Most pituitary tumors that occur in the mouse and rat in chronic toxicity/carcinogenicity studies occur late in these studies and are preceded by hyperplasia indicating that escape from hypothalamic inhibition or excessive exposure to stimulating hormone may function as tumor promoters. Indeed, the significant decrease in pituitary adenomas in diet-restricted female B6C3F1 mice is speculated to be a consequence of decreased production of hypothalamic neurotransmitters (Sheldon et al. 1995). However, the latency for pituitary tumor development in mice suggests that multiple factors may be necessary for tumorigenesis.
Pars Distalis
Hyperplasia
Hyperplasia of the pars distalis may occur as a focal or diffuse increase in the number of cells. Focal hyperplasia is usually not sharply demarcated, may have prominent dilated vascular sinusoids, and typically does not cause compression of adjacent parenchyma. Diffuse hyperplasia is often difficult to assess and is usually recognized as a symmetrical enlargement of the pars distalis (Greaves 2012). Focal hyperplasia and unilateral hyperplasia are more readily identified in the mouse pars distalis (Figure 3, Figure 13, Figure 14, & Figure 15). As is the case with other endocrine tissues, there is a continuum between hyperplasia and neoplasia of the pars distalis that can create challenges in reaching consensus diagnoses among pathologists. Increased pituitary weight is supportive of a diagnosis of diffuse hyperplasia.
Bielschowsky et al. (1956) reported on pituitary gland hyperplasia with prolactin-secreting nodule formation occurring in intact NZY female mice with spontaneous mammary gland tumors. Similarly, physiological hyperplasia of prolactin-secreting pituitary cells has been associated with spontaneously occurring mammary gland hyperplasia in virgin female FVB/NCr mice with some mice developing pituitary adenomas (Wakefield et al. 2003).
Adenoma
Adenomas of the pars distalis are discrete focal lesions consisting of a monomorphic population of cells causing compression of adjacent pituitary structures (Figures 16, 17, 18, & 19). Their size is generally greater than 50% of the pituitary height (Capen et al. 2001). Cells comprising the adenoma may be enlarged but are relatively homogeneous. Growth pattern may be trabecular, pseudofollicular, in nests, or solid often with angiectasis and sometimes with hemorrhage. Most pars distalis adenomas in mice are prolactinomas (Liebelt 1994). Offspring of CD-1 mice exposed to diethylstibesterol late in pregnancy develop prolactinomas at approximatedly 20 months of age (Walker and Kurth 1993). Monomorphic transplantable ACTH-secreting pituitary tumors were induced in LAF1 mice following exposure at ground zero to ionizing radiation from an atomic denotation (Furth 1955). Spontaneous adenohypophysis tumors occur at two years of age in LAF1 mice but with reduced latency following ionizing radiation with greater frequency in females (Upton & Furth 1955).
Carcinoma
Pars distalis carcinomas are rare in mice and share many features with adenomas, especially angiectasis and hemorrhage with associated hematogenous pigment and presence of macrophages. Hallmarks for malignancy include cellular pleomorphism and atypia, sometimes with giant cells, and definitive infiltration outside the pituitary (Figures 20-22).
Pars Intermedia
Hyperplasia
Hyperplasia occurs as a focal increase of uniform cells or as a diffuse increase in volume of the pars intermedia. Because of the position of the pars intermedia, hyperplasia may extend by expansion into the pars distalis and pars nervosa without causing compression.
Adenoma
Adenoma of the pars intermedia is comprised of a focal increase in pale staining cells that may be tinctorially different from the adjacent parenchyma with some pleomorphism (Figures 23-27). Diagnostic features include loss of the lobular pattern of growth and compression of surrounding tissues.
Carcinoma
Pars intermedia carcinomas (Figures 28 & 29) are rare in mice and have similar features to pars intermedia adenomas. Diagnosis of malignancy is based on actual infiltration outside the pituitary.
Pituicytoma
Pituicytomas originate from the neurohypophysis and consist of tightly packed sheets of spindeloid cells with elongated nuclei with finely stippled chromatin, small nucleoli, and foamy to vacuolated eosinophilic cytoplasm and indistinct cell borders (Tekeli et al. 1997). Small sheets and clusters of tumor cells are separated by GFAP-positive neurophil. Compression of adjacent pituitary tissue may be evident and occasional nuclear palisading has been described (Tekeli et al. 1997; Capen et al. 2001).
Craniopharyngioma
Craniopharyngiomas arise from remnants of the oropharyngeal epithelium of craniopharyngeal duct (Rathke’s pouch) and consist of proliferating keratinized epithelium with hyperkeratosis and parakeratosis and display a variety of growth patterns. These neoplasms are considered benign when they are not truly infiltrating adjacent tissues. Diagnosis of malignancy is based on distinct infiltration of the brain.
Other Tumors
Metastatic and systemic neoplasia that may involve the mouse pituitary gland include histiocytic sarcoma (Figure 30), erythroleukemia (Figures 31 & 32), and meningioma.
Mouse Pituitary Models
Spontaneous and genetically modified mouse models have elucidated the role of genes and transcription factors in pituitary ontogeny, dysfunction and neoplasia. In general, the various mutant mouse models share important features with human endocrine deficiencies (Keegan & Camper 2003). The Mendelian recessive dwarf mouse, originally described in 1929 (Snell 1929), is possibly the oldest mouse pituitary model and presents with an underdeveloped pituitary as a primary defect with secondary hypoplastic effects on adrenals, thyroids, and gonads (Snell 1929; Bartke 1964), thereby revealing a master gland function for the pituitary gland. Confirmation that the dwarf mouse does not synthesize and secrete growth hormone, prolactin, and thyroid stimulating hormone has been confirmed by electrophoresis, special staining, and immunohistochemistry (Roux et al. 1982; Lewis et al. 1965; Cheever et al. 1969; Elftman & Wegelius 1959). Subsequent to identification of the 1929 Snell dwarf mouse, a second dwarf mouse with a single nucleotide mutation in the prophet of pituitary factor (prop1) gene, a transcription factor responsible for pituitary development, was described (Barke 1964; Brown-Borg et al. 1996; Armstrong et al. 2014; Victoria et al. 2015). These two dwarf mutants as well as genetically modified dwarf mouse models, all characteristically long-lived, have reduced activity of the somatotrophic axis and are of keen interest in geriatric research (Bartke & Brown-Borg 2004).
Various mouse models of pituitary hyperplasia and neoplasia have been developed using trophic-specific alterations and following radiation exposure. Studies in the mid-1900s reported on differential strain susceptibility in development of pituitary tumors following estrogen exposure (Garner & Strong 1940), the production of pituitary chromophobe adenomas in mice following implantation of diethylstibesterol pellets (Woolley & Little 1946), basophilic pituitary tumors following early gonadectomy (Dickey and Lane 1956; Dickie & Woolley 1949), estrogen-induced transplantable pituitary tumors that secreted both growth hormone and prolactin (Furth et al., 1956), and radiation induced pituitary tumors that secreted prolactin and ACTH (Upton & Furth 1955; Furth et al., 1953).
A variety of transgenic mouse models of pituitary neoplasia have been reported. The steroidogenic factor 1 (SF1) knock out mouse serves as an animal model of hypogonadodrophic hypogonadism and has established an important function of SF1 in pituitary gonadotrophs (Zhao et al. 2001). Adenohypophysial changes in mice transgenic for human growth hormone-releasing factor (hGFR) have been investigated using conventional histopathology, immunohistochemistry, in situ hybridization and electron microscopy and include hyperplasia of somatotrophs, lactotrophs, and mammosomatotrophs within 6 to 8 months with subsequent development of adenomas as early as 10 months (Stefaneanu et al. 1989; Asa et al. 1992; Mayo et al. 1988). These hGFR mouse models provide direct evidence that a neuropeptide can act as a trophic factor on pituitary cells and show that the chronic stimulation by hGFR leads to paracrine, endocrine, and autocrine mechanisms driving pituitary cell proliferation (Mayo et al. 1988; Lloyd et al. 1992). Transgenic mice with fusion of bovine arginine vasopressin and SV40-large T antigen were studied using conventional histopathology, immunohistochemistry and electron microscopy and were shown to develop pars distalis adenomas consisting of undifferentiated somatotrophs as well as pancreatic insulinomas (Stefaneanu et al. 1992).
Hypothalamic dopamine acts on dopamine D2 receptors in pituitary lactotrophs to tonically inhibit prolactin expression and secretion. Chronic loss of this neurohormonal inhibition in dopamine D2 receptor deficient mice results in persistent hyperprolactemia with lactotroph hyperplasia and adenomas, including lactotroph adenomas in male mice (Asa et al. 1999; Kelly et al. 1997). Mice with SV40-Large T antigen fused to human glycoprotein hormone develop anterior pituitary tumors of gonadotrophic lineage (Windle et al. 1990) while corticotrophic tumors of the pituitary in a mouse model of Cushing’s disease are produced in polyoma large T antigen transgenic mice (Helseth et al. 1992). Both corticotrophic and melanotrophic adenomas developed in neuron-specific proopiomelanocortin-deficient mice (Smart et al. 2006). These and other mouse pituitary models developed through genetic modification have helped define the molecular underpinnings of hypothalamic/pituitary function. Pituitary mouse models are the subject of recent reviews (Cushman & Camper 2001; Cano et al. 2014; Bartke & Brown-Borg 2004).
Practical Notes
Given the small size of the mouse pituitary, it is recommended that it be fixed in situ to avoid artifacts that may occur when removed fresh and unfixed. Weighing pituitary after fixation is practical. Pituitary weights may be a more accurate assessment of hyperplasia or hypertrophy than histopathology, especially when microscopic evidence is minimal.
Separate embedding or embedding along with adrenals will generally increase the chances for the microtomist to obtain a more complete histological representation of pituitary components (pars distalis, pars intermedia, and pars nervosa).
Definitive identification of acidophils, basophils, and chromophobes and their relative proportions in hematoxylin & eosin-stained sections is difficult and may not be practical or accurate. Definitive identification of these cell types requires immunohistochemistry.
As there is a continuum in the morphological changes from hyperplasia to neoplasia, it is recommended that all hyperplastic lesions be given a severity grade when evaluating safety assessment studies.
The pituitary gland is considered a master endocrine gland that influences as well as reflects changes in other endocrine and related reproductive tissues. Consequently, assessments of pituitary gland features are ideally evaluated in conjunction with changes in other endocrine and related reproductive tissues.
References
Allaerts, W., Jeucken, P. H., Bosman, F. T., and Drexhage, H. A. (1993). Relationship between dendritic cells and folliculo-stellate cells in the pituitary: immunohistochemical comparison between mouse, rat and human pituitaries. Adv Exp Med Biol 329, 637-42.
Allaerts, W., Jeucken, P. H., Hofland, L. J., and Drexhage, H. A. (1991). Morphological, immunohistochemical and functional homologies between pituitary folliculo-stellate cells and lymphoid dendritic cells. Acta Endocrinol (Copenh) 125 Suppl 1, 92-7.
Andoniadou, C. L., Matsushima, D., Mousavy Gharavy, S. N., Signore, M., Mackintosh, A. I., Schaeffer, M., Gaston-Massuet, C., Mollard, P., Jacques, T. S., Le Tissier, P., Dattani, M. T., Pevny, L. H., and Martinez-Barbera, J. P. (2013). Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 13, 433-45.
Armstrong, V. L., Rakoczy, S., Rojanathammanee, L., and Brown-Borg, H. M. (2014). Expression of DNA methyltransferases is influenced by growth hormone in the long-living Ames dwarf mouse in vivo and in vitro. J Gerontol A Biol Sci Med Sci 69, 923-33.
Asa, S. L., and Ezzat, S. (1999). Molecular determinants of pituitary cytodifferentiation. Pituitary 1, 159-68.
Asa, S. L., Kelly, M. A., Grandy, D. K., and Low, M. J. (1999). Pituitary lactotroph adenomas develop after prolonged lactotroph hyperplasia in dopamine D2 receptor-deficient mice. Endocrinology 140, 5348-55.
Asa, S. L., Kovacs, K., Stefaneanu, L., Horvath, E., Billestrup, N., Gonzalez-Manchon, C., and Vale, W. (1992). Pituitary adenomas in mice transgenic for growth hormone-releasing hormone. Endocrinology 131, 2083-9.
Bartke, A. (1964). Histology of the anterior hypophysis, thyroid and gonads of two types of dwarf mice. Anat Rec 149, 225-236.
Bartke, A., and Brown-Borg, H. (2004). Life extension in the dwarf mouse. Curr Top Dev Biol 63, 189-225.
Bielschowsky, M., Bielschowsky, F., and Lindsay, D. (1956). A new strain of mice with a high incidence of mammary cancers and enlargement of the pituitary. Br J Cancer 10, 688-99.
Blumenthal, H. T. (1955). Aging processes in the endocrine glands of various strains of normal mice: relationship of hypophyseal activity to aging changes in other endocrine glands. J Gerontol 10, 253-67.
Brown, C. H. (2016). Magnocellular Neurons and Posterior Pituitary Function. Compr Physiol 6, 1701-1741.
Brown-Borg, H. M., Borg, K. E., Meliska, C. J., and Bartke, A. (1996). Dwarf mice and the ageing process. Nature 384, 33.
Cameron, A., and Sheldon, W. (1999). Cystoid degeneration, anterior pituitary, mouse. In Endocrine System (T. Jones, U. Mohr and R. Hunt, eds.). Springer-Verlag, New York.
Cano, D. A., Soto-Moreno, A., and Leal-Cerro, A. (2014). Genetically engineered mouse models of pituitary tumors. Front Oncol 4, 203.
Capen, C. (1996). Functional and pathological interrelationships of the pituitary gland and hypothalamus in animals. In Monographs on Pathology of Laboaratory Animals. Endocrine System (T. Jones, C. Capen and U. Mohr, eds.), pp. 3-33. Springer, Berlin.
Capen, C., DeLellis, R., and Yarrington, J. (1991). Endocrine system. In Handbook of Toxicologic Pathology (W. Haschek and C. Rousseaux, eds.). Academic Press, San Diego.
Capen, C., Karbe, E., and Etc. (2001). Endocrine system. In International Classification of Rodent Tumors. The Mouse (U. Mohr, ed.) Springer, Amsterdam.
Cheever, E. V., Seavey, B. K., and Lewis, U. J. (1969). Prolactin of normal and dwarf mice. Endocrinology 85, 698-703.
Cushman, L. J., and Camper, S. A. (2001). Molecular basis of pituitary dysfunction in mouse and human. Mamm Genome 12, 485-94.
Dasen, J. S., Martinez Barbera, J. P., Herman, T. S., Connell, S. O., Olson, L., Ju, B., Tollkuhn, J., Baek, S. H., Rose, D. W., and Rosenfeld, M. G. (2001). Temporal regulation of a paired-like homeodomain repressor/TLE corepressor complex and a related activator is required for pituitary organogenesis. Genes Dev 15, 3193-207.
Dasen, J. S., O’Connell, S. M., Flynn, S. E., Treier, M., Gleiberman, A. S., Szeto, D. P., Hooshmand, F., Aggarwal, A. K., and Rosenfeld, M. G. (1999). Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell 97, 587-98.
Dasen, J. S., and Rosenfeld, M. G. (1999). Signaling mechanisms in pituitary morphogenesis and cell fate determination. Curr Opin Cell Biol 11, 669-77.
Dasen, J. S., and Rosenfeld, M. G. (2001). Signaling and transcriptional mechanisms in pituitary development. Annu Rev Neurosci 24, 327-55.
Dickie, M. M., and Lane, P. W. (1956). Adrenal tumors, pituitary tumors, and other pathological changes in F hybrids of strain DE x strain DBA. Cancer Res 16, 48-52.
Dickie, M. M., and Woolley, G. W. (1949). Spontaneous basophilic tumors of the pituitary glands in gonadectomized mice. Cancer Res 9, 372-84.
Dubois, P. M., and Elamraoui, A. (1995). Embryology of the pituitary gland. Trends Endocrinol Metab 6, 1-7.
Elftman, H., and Wegelius, O. (1959). Anterior pituitary cytology of the dwarf mouse. Anat Rec 135, 43-9.
Fauquier, T., Guerineau, N. C., McKinney, R. A., Bauer, K., and Mollard, P. (2001). Folliculostellate cell network: a route for long-distance communication in the anterior pituitary. Proc Natl Acad Sci U S A 98, 8891-6.
Fauquier, T., Rizzoti, K., Dattani, M., Lovell-Badge, R., and Robinson, I. C. (2008). SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci U S A 105, 2907-12.
Florio, T. (2011). Adult pituitary stem cells: from pituitary plasticity to adenoma development. Neuroendocrinology 94, 265-77.
Frith, C., and Ward, J. (1988). Color Atlas of Neoplastic and Non-Neoplastic Lesions in Aging MICe. Elsevier, Amsterdam.
Furth, J., Gadsen, E. L., and Upton, A. C. (1953). ACTH secreting transplantable pituitary tumors. Proc Soc Exp Biol Med 84, 253-4.
Gardner, W. U., and Strong, L. C. (1940). Strain-limited Development of Tumors of the Pituitary Gland in Mice Receiving Estrogens. Yale J Biol Med 12, 543-548 1.
Greaves, P. (2012). Histopathology of preclinical toxicity studies. Elsevier, Amsterdam.
Hashimoto, H., Ishikawa, H., and Kusakabe, M. (1998). Three-dimensional analysis of the developing pituitary gland in the mouse. Dev Dyn 212, 157-66.
Hatton, G. I. (1988). Pituicytes, glia and control of terminal secretion. J Exp Biol 139, 67-79.
Heathcote, G. M., Bromage, T. G., Sava, V. J., Hanson, D. B., and Anderson, B. E. (2014). Enigmatic cranial superstructures among Chamorro ancestors from the Mariana Islands: gross anatomy and microanatomy. Anat Rec (Hoboken) 297, 1009-21.
Helseth, A., Siegal, G. P., Haug, E., and Bautch, V. L. (1992). Transgenic mice that develop pituitary tumors. A model for Cushing’s disease. Am J Pathol 140, 1071-80.
Inoue, K., and Kurosumi, K. (1981). Mode of proliferation of gonadotrophic cells of the anterior pituitary after castration–immunocytochemical and autoradiographic studies. Arch Histol Jpn 44, 71-85.
Jarskar, R. (1977). Electron microscopical study on the development of the nerve supply of the pituitary pars intermedia of the mouse. Cell Tissue Res 184, 121-32.
Jilg, A., Moek, J., Weaver, D. R., Korf, H. W., Stehle, J. H., and von Gall, C. (2005). Rhythms in clock proteins in the mouse pars tuberalis depend on MT1 melatonin receptor signalling. Eur J Neurosci 22, 2845-54.
Jin, L., Zhang, S., Burguera, B. G., Couce, M. E., Osamura, R. Y., Kulig, E., and Lloyd, R. V. (2000). Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology 141, 333-9.
Kaufman, M. (1994). The atlas of mouse development. Academic Press, London.
Kaufman, M., Nikitin, A., and Sundberg, J. (2010). Histologic basis of mouse endocrine system development. CRC Press, Boca Raton.
Keegan, C. E., and Camper, S. A. (2003). Mouse knockout solves endocrine puzzle and promotes new pituitary lineage model. Genes Dev 17, 677-82.
Kelberman, D., and Dattani, M. T. (2007). Hypothalamic and pituitary development: novel insights into the aetiology. Eur J Endocrinol 157 Suppl 1, S3-14.
Kelberman, D., Rizzoti, K., Lovell-Badge, R., Robinson, I. C., and Dattani, M. T. (2009). Genetic regulation of pituitary gland development in human and mouse. Endocr Rev 30, 790-829.
Kelly, M. A., Rubinstein, M., Asa, S. L., Zhang, G., Saez, C., Bunzow, J. R., Allen, R. G., Hnasko, R., Ben-Jonathan, N., Grandy, D. K., and Low, M. J. (1997). Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19, 103-13.
Keough, E. M., and Wood, B. G. (1978). The effects of adrenalectomy and cortisol treatment on cell types, other than corticotrophs, in the anterior pituitary of the mouse. Tissue Cell 10, 563-70.
Kerr, T. (1946). The development of the pituitary of the laboratory mouse. Q.J. Microsc. Sci. 87, 3-29.
Levy, A. (2002). Physiological implications of pituitary trophic activity. J Endocrinol 174, 147-55.
Levy, A. (2008). Stem cells, hormones and pituitary adenomas. J Neuroendocrinol 20, 139-40.
Lewis, U. J., Cheever, E. V., and Vanderlaan, W. P. (1965). Studies on the Growth Hormone of Normal Dwarf Mice. Endocrinology 76, 210-5.
Liebelt, A. G. (1994). Tumours of the pituitary gland. IARC Sci Publ, 527-63.
Lloyd, R. V., Jin, L., Chang, A., Kulig, E., Camper, S. A., Ross, B. D., Downs, T. R., and Frohman, L. A. (1992). Morphologic effects of hGRH gene expression on the pituitary, liver, and pancreas of MT-hGRH transgenic mice. An in situ hybridization analysis. Am J Pathol 141, 895-906.
Lupi, I., Zhang, J., Gutenberg, A., Landek-Salgado, M., Tzou, S. C., Mori, S., and Caturegli, P. (2011). From pituitary expansion to empty sella: disease progression in a mouse model of autoimmune hypophysitis. Endocrinology 152, 4190-8.
Mahler, J., and Elwell, M. (1999). Pituitary gland. In Pathoogy of the Mouse (R. Maronpot, ed. Cache River Press, Vienna, IL.
Mayo, K. E., Hammer, R. E., Swanson, L. W., Brinster, R. L., Rosenfeld, M. G., and Evans, R. M. (1988). Dramatic pituitary hyperplasia in transgenic mice expressing a human growth hormone-releasing factor gene. Mol Endocrinol 2, 606-12.
Morgan, P. J., and Williams, L. M. (1996). The pars tuberalis of the pituitary: a gateway for neuroendocrine output. Rev Reprod 1, 153-61.
Morton, D., and Tekeli, S. (1997). “Have you seen this?” Pituitary cysts in a mouse. Toxicol Pathol 25, 333.
Nolan, L. A., Kavanagh, E., Lightman, S. L., and Levy, A. (1998). Anterior pituitary cell population control: basal cell turnover and the effects of adrenalectomy and dexamethasone treatment. J Neuroendocrinol 10, 207-15.
Nunez, L., Villalobos, C., Senovilla, L., and Garcia-Sancho, J. (2003). Multifunctional cells of mouse anterior pituitary reveal a striking sexual dimorphism. J Physiol 549, 835-43.
Oishi, Y., Matsumoto, M., Yoshizawa, K., Tsubura, A., and Morii, S. (1992). Spontaneous pituitary adenomas of the pars intermedia in mice and rats: Histopathological and immunocytochemical studies. J. Toxicol Pathol 5, 223-231.
Ranadive, K. J., and Karande, K. A. (1970). Pituitaries of spayed mice of different strains: cytology and gonadotrophin content. J Endocrinol 48, 449-56.
Rizzoti, K. (2015). Genetic regulation of murine pituitary development. J Mol Endocrinol 54, R55-73.
Rizzoti, K., Akiyama, H., and Lovell-Badge, R. (2013). Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell 13, 419-32.
Rizzoti, K., and Lovell-Badge, R. (2005). Early development of the pituitary gland: induction and shaping of Rathke’s pouch. Rev Endocr Metab Disord 6, 161-72.
Roux, M., Bartke, A., Dumont, F., and Dubois, M. P. (1982). Immunohistological study of the anterior pituitary gland – pars distalis and pars intermedia – in dwarf mice. Cell Tissue Res 223, 415-20.
Roux, M., and Dubois, M. P. (1976). Immunohistochemical study of the pars intermediate of the mouse pituitary in different experimental conditions. Experientia 32, 657-8.
Samson, W. K., and Schell, D. A. (1995). Oxytocin and the anterior pituitary gland. Adv Exp Med Biol 395, 355-64.
Sano, M., and Sasaki, F. (1969). Embryonic development of the mouse anterior pituitary studied by light and electron microscopy. Z Anat Entwicklungsgesch 129, 195-222.
Sasaki, F., and Iwama, Y. (1988). Sex difference in prolactin and growth hormone cells in mouse adenohypophysis: stereological, morphometric, and immunohistochemical studies by light and electron microscopy. Endocrinology 123, 905-12.
Sasaki, F., Iwama, Y., and Sano, M. (1979). Strain-difference in prolactin cells of mouse anterior pituitary between high and low mammary tumor strains by stereological morphometry with an electron microscope. Okajimas Folia Anat Jpn 55, 341-50.
Schaeffer, M., Hodson, D. J., Lafont, C., and Mollard, P. (2010). Functional importance of blood flow dynamics and partial oxygen pressure in the anterior pituitary. Eur J Neurosci 32, 2087-95.
Senovilla, L., Nunez, L., Villalobos, C., and Garcia-Sancho, J. (2008). Rapid changes in anterior pituitary cell phenotypes in male and female mice after acute cold stress. Endocrinology 149, 2159-67.
Sheldon, W. G., Bucci, T. J., Hart, R. W., and Turturro, A. (1995). Age-related neoplasia in a lifetime study of ad libitum-fed and food-restricted B6C3F1 mice. Toxicol Pathol 23, 458-76.
Smart, J. L., Tolle, V., Otero-Corchon, V., and Low, M. J. (2007). Central dysregulation of the hypothalamic-pituitary-adrenal axis in neuron-specific proopiomelanocortin-deficient mice. Endocrinology 148, 647-59.
Snell, G. D. (1929). Dwarf, a New Mendelian Recessive Character of the House Mouse. Proc Natl Acad Sci U S A 15, 733-4.
Stefaneanu, L., Kovacs, K., Lloyd, R. V., Scheithauer, B. W., Young, W. F., Jr., Sano, T., and Jin, L. (1992). Pituitary lactotrophs and somatotrophs in pregnancy: a correlative in situ hybridization and immunocytochemical study. Virchows Arch B Cell Pathol Incl Mol Pathol 62, 291-6.
Takuma, N., Sheng, H. Z., Furuta, Y., Ward, J. M., Sharma, K., Hogan, B. L., Pfaff, S. L., Westphal, H., Kimura, S., and Mahon, K. A. (1998). Formation of Rathke’s pouch requires dual induction from the diencephalon. Development 125, 4835-40.
Tzou, S. C., Lupi, I., Landek, M., Gutenberg, A., Tzou, Y. M., Kimura, H., Pinna, G., Rose, N. R., and Caturegli, P. (2008). Autoimmune hypophysitis of SJL mice: clinical insights from a new animal model. Endocrinology 149, 3461-9.
Upton, A. C., and Furth, J. (1953). Induction of pituitary tumors by means of ionizing irradiation. Proc Soc Exp Biol Med 84, 255-7.
Upton, A. C., and Furth, J. (1955). Spontaneous and radiation-induced pituitary adenomas of mice. J Natl Cancer Inst 15, 1005-21.
Vankelecom, H. (2010). Pituitary stem/progenitor cells: embryonic players in the adult gland? Eur J Neurosci 32, 2063-81.
Vankelecom, H., Matthys, P., Van Damme, J., Heremans, H., Billiau, A., and Denef, C. (1993). Immunocytochemical evidence that S-100-positive cells of the mouse anterior pituitary contain interleukin-6 immunoreactivity. J Histochem Cytochem 41, 151-6.
Victoria, B., Dhahbi, J. M., Nunez Lopez, Y. O., Spinel, L., Atamna, H., Spindler, S. R., and Masternak, M. M. (2015). Circulating microRNA signature of genotype-by-age interactions in the long-lived Ames dwarf mouse. Aging Cell 14, 1055-66.
Vidal, S., Horvath, E., Kovacs, K., Lloyd, R. V., and Smyth, H. S. (2001). Reversible transdifferentiation: interconversion of somatotrophs and lactotrophs in pituitary hyperplasia. Mod Pathol 14, 20-8.
Wakefield, L. M., Thordarson, G., Nieto, A. I., Shyamala, G., Galvez, J. J., Anver, M. R., and Cardiff, R. D. (2003). Spontaneous pituitary abnormalities and mammary hyperplasia in FVB/NCr mice: implications for mouse modeling. Comp Med 53, 424-32.
Walker, B. E., and Kurth, L. A. (1993). Pituitary tumors in mice exposed prenatally to diethylstilbestrol. Cancer Res 53, 1546-9.
Watanabe, Y. G. (1991). The occurrence and developmental origin of epithelial cysts in the rat and mouse adenohypophysis. Arch Histol Cytol 54, 511-8.
Windle, J. J., Weiner, R. I., and Mellon, P. L. (1990). Cell lines of the pituitary gonadotrope lineage derived by targeted oncogenesis in transgenic mice. Mol Endocrinol 4, 597-603.
Woolley, G. W., and Little, C. C. (1946). Prevention of Adrenal Cortical Carcinoma by Diethylstilbestrol. Proc Natl Acad Sci U S A 32, 239-40.
Yamada, K., Sano, M., and Ito, T. (1957). A postnatal histogenetic study of the anterior pituitary of the mouse. Okajimas Folia Anat Jpn 30, 177-95.
Zhao, L., Bakke, M., and Parker, K. L. (2001). Pituitary-specific knockout of steroidogenic factor 1. Mol Cell Endocrinol 185, 27-32.
Zhu, X., Gleiberman, A. S., and Rosenfeld, M. G. (2007). Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87, 933-63.