Quercetin and its glycosides possess potential benefits to human health. Several flavonols are available to consumers as dietary supplements, promoted as anti-oxidants; however, incorporation of natural quercetin glycosides into food and beverage products has been limited by poor miscibility in water. Enzymatic conjugation of multiple glucose moieties to isoquercitrin to produce alpha-glycosyl isoquercitrin (AGIQ) enhances solubility and bioavailability. AGIQ is used in Japan as a food additive and has been granted generally recognized as safe (GRAS) status. However, although substantial genotoxicity data exist for quercetin, there is very little available data for AGIQ and isoquercitrin. To support expanded global marketing of food products containing AGIQ, comprehensive testing of genotoxic potential of AGIQ and isoquercitrin was conducted according to current regulatory test guidelines. Both chemicals tested positive in bacterial reverse mutation assays, and exposure to isoquercitrin resulted in chromosomal aberrations in CHO-WBL cells. All other in vitro mammalian micronucleus and chromosomal aberration assays, micronucleus and comet assays in male and female B6C3F1 mice and Sprague Dawley rats, and Muta™ Mouse mutation assays evaluating multiple potential target tissues, were negative for both chemicals. These results supplement existing toxicity data to further support the safe use of AGIQ in food and beverage products.
1. Introduction
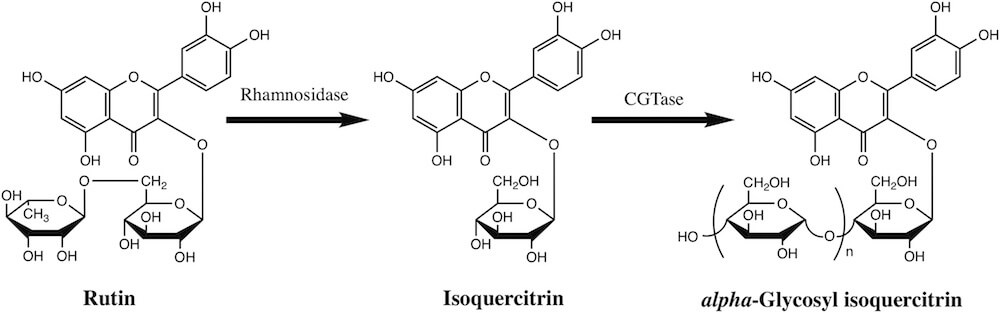
Isoquercitrin (quercetin-3-O-β-D-glucoside) is a natural flavonoid that is purported to have anti-inflammatory, anti-oxidant, anti-mutagenic, anti-clastogenic, anti-depressant, hypotensive, hypolipidemic and anti-viral properties (Amado et al., 2009; Edenharder et al., 2003; Gasparotto Junior et al., 2011; Kim et al., 2010; Li et al., 2011; Valentova et al., 2014). Large volumes of isoquercitrin can be manufactured via enzymatic hydrolysis of rutin and then transglycosylated using dextrin and cyclodextrin glucanotransferase (CGTase) to produce alpha-glycosyl isoquercitrin (AGIQ), also known as enzymatically modified isoquercitrin (EMIQ) (Fig. 1) (Erlund, 2004; Manach et al., 1997). The conjugation of multiple glucose moieties confers increased solubility in water and substantially greater bioavailabilityof AGIQ, which may contribute to its potent pharmacological activities (Makino et al., 2009), including anti-oxidant (Kangawa et al., 2017a; Morita et al., 2011; Nishimura et al., 2010; Shimada et al., 2010; Yoshida et al., 2017) and tumor suppressive (Fujii et al., 2013; Hara et al., 2014; Kangawa et al., 2017b; Kimura et al., 2013) effects in animals. It is used primarily in Japan as an additive in beverages, wine coolers, frozen dairy products, gelatins and puddings, jams and jellies, soft candy, baked desserts, powdered or canned soup and chewing gum. Both AGIQ and isoquercitrin are directly available to consumers as dietary supplements, promoted for their anti-oxidant properties.
Fig. 1. Enzymatic production of isoquercitrin and alpha-glycosyl isoquercitrin. Rutin isolated from buds and flowers of Sophora japonica is converted to isoquercitrin using rhamnosidase. Cyclodextrin glucanotransferase (CGTase), purified from Bacillus pseudalcaliphilus DK-1139, catalyzes cleavage of glycosidic bonds to modify isoquercitrin by addition of multiple dextrin molecules to form alpha-glycosyl isoquercitrin.
AGIQ has been consumed in Japan since 1987 and was approved by the Japanese Ministry of Health and Welfare for use as a food additive in 1996 (http://www.ffcr.or.jp/zaidan/FFCRHOME.nsf/pages/list-exst.add), granted generally recognized as safe (GRAS) status by the Expert Panel of the Flavor and Extract Manufacturers Association (FEMA) in 2005 (Smith et al., 2005a, 2005b), and GRAS status by the U.S. Food and Drug Administration (FDA) as an anti-oxidant for food and beverage products in 2007 (FDA, 2007). AGIQ was shown to be safe and non-carcinogenic in previous toxicological studies (Hasumura et al., 2004; Salim et al., 2004; Tamano et al., 2001). However, the safety and toxicity information for isoquercitrin and AGIQ is sparse and the published safety assessment reports are based on older non-Good Laboratory Practice (GLP)-compliant studies and/or studies that used test substance of low purity (Engen et al., 2015; Salim et al., 2004; Valentova et al., 2014). Although fairly extensive genotoxicity data exist for quercetin (Engen et al., 2015; Harwood et al., 2007; Utesch et al., 2008), a decomposition product and minor constituent of AGIQ, there is minimal available information on the genotoxic potential of AGIQ and isoquercitrin.
Given the limitations in currently available data, in anticipation of expanded global marketing of food products containing AGIQ, we evaluated highly purified AGIQ in a GLP-compliant test battery in accordance with current EFSA, OECD, and FDA guidances on genotoxicity and toxicity testing (EFSA, 2010, 2011; FDA, 2000a,b,c; OECD, 1997, 1998, 2010, 2011, 2014a,b,c,d, 2016). As an intermediate molecule in the enzymatic synthesis of AGIQ, the genotoxic potential of isoquercitrin was also evaluated. We recently reported the results of 90-day repeat dose toxicity and single dose toxicokinetic studies of AGIQ (Nyska et al., 2016). The results of the comprehensive assessment of genotoxicity are reported here, including bacterial reverse mutation assays, in vitro mammalian micronucleus and chromosome aberration assays, combined micronucleus/comet assays conducted in male and female B6C3F1 mice and Sprague Dawley rats, and transgenic mouse (Muta™ Mouse) mutation assays.
2. Material and methods
2.1. Chemical analysis
All genotoxicity assays were conducted in accordance with OECD guidelines and were GLP-compliant. AGIQ [≥97% pure (0.10% quercetin)] and isoquercitrin (≥95% pure; CAS No. 482-35-9) were provided by San-Ei Gen F.F.I., Inc., Osaka, Japan. AGIQ was prepared from rutin, an extract from the buds or flowers of the Sophora japonica Linné plant (also known as the Japanese pagoda tree), according to the specifications outlined in the 8th edition of the Japanese Specifications and Standards for Food Additives (http://www.ffcr.or.jp/zaidan/FFCRHOME.nsf/pages/spec.stand.fa); the material tested is the same material used in commercially available food products. Test chemicals were dissolved in sterile water or DMSO, suspended in corn oil, or blended into rodent chow, as indicated below for each assay. Samples removed from the top, middle, and bottom fractions of each chemical formulation were submitted for analytical testing (Alera Laboratories, LLC, Durham, NC; BSRC, Shizuoka, Japan). Prepared diets were sampled just after preparation for stability/homogeneity/concentration analysis performed at the BSRC; prepared diets were stored at room temperature and used within 17 (isoquercitrin) or 14 days (AGIQ) after preparation. All analyzed dose formulations were within 15% of nominal concentrations and were determined to be stable over the course of the experiments.
2.2. Bacterial reverse mutation assay
Mutagenicity assays of isoquercitrin and AGIQ, with and without metabolic activation, were conducted as described previously (Ames et al., 1975; Gatehouse et al., 1994; Maron and Ames, 1983; Mortelmans and Zeiger, 2000) and according to the guideline for the bacterial reverse mutation assay (OECD 471). The S. typhimurium strains TA100, TA98, TA1535 and TA1537 were originally obtained from Dr. Bruce Ames (University of California) and the E. coli WP2 uvrA strain from the National Institute of Hygienic Science. All strains were checked for maintenance of genetic markers prior to the study. Metabolic activation was provided by a 10% phenobarbital/benzoflavone-induced rat liver S9 mix (Kikkoman Co., Noda, Japan) with added cofactors. Based on the results of a range-finding assay (Supplemental Data Table S1), isoquercitrin was tested at a top concentration of 5000 μg/plate (limit dose) in TA1535 and E. coli WP2 uvrA in the presence and absence of metabolic activation (±S9) and TA100 and TA98 in the absence of metabolic activation (–S9). A top concentration of 2500 μg/plate isoquercitrin was tested in TA100, TA98 and TA1537 in the presence of metabolic activation (+S9) and 1250 μg/plate in TA1537 –S9. Since no toxicity was observed in a range-finding assay of AGIQ (Supplemental Data Table S2), a top dose of 5000 μg/plate was selected for all strains ± S9. AGIQ and isoquercitrin were dissolved in water for injection and DMSO, respectively. Positive controls tested without metabolic activation were 2-(2-furyl)-3-(5-nitro-2-furyl) acrylamide (TA100, TA98, E. coli WP2), sodium azide (TA1535) and 9-aminoacridine hydrochloride (TA1537); 2-aminoanthracene was tested in all strains with metabolic activation. The assay tubes were pre-incubated at 37 °C for 20 min before plating onto minimum glucose agar. Three test plates per concentration were incubated at 37 °C for 48 h and then counted using an automated colony analyzer (CA-11, System Science Co., Ltd., Tokyo, Japan), correcting for the area and count loss.
2.3. In vitro micronucleus (MN) assay
2 MN assays were conducted using human TP53 competent TK6 cells (ATCC, Manassas, VA) according to OECD 487 (OECD, 2014a) as described previously (Hobbs et al., 2015). Triplicate cultures of exponentially growing cells seeded at 2.0 ± 0.25 × 105 cells/mL in 12-well plates were exposed to AGIQ, isoquercitrin, or controls for 4 h in the presence and absence of metabolic activation (±S9) and 24 h in the absence of metabolic activation (–S9). The composition of the S9 mix was: 10% S9, 8 mM MgCl2, 32.6 mM KCl, 4.7 mM glucose-6-phosphate, 4 mM NADP, 0.1 M phosphate buffer; the final concentration of S9 in the cultures was 1%. Cyclophosphamide and vinblastine in DMSO were used as the positive controls with and without metabolic activation, respectively. Based on a dose setting toxicity test (Supplemental Data Table S3), the concentrations of AGIQ selected for testing were 5000 (limit dose), 2500, 1250, 625, 313 and 156 μg/mL prepared in sterile water for all test conditions. The results of toxicity tests for isoquercitrin revealed that the concentrations required to produce the recommended level of cytotoxicity at the top dose to be different for the different metabolic test conditions (Supplemental Data Table S4). On this basis, the top concentrations of isoquercitrin (prepared in DMSO) selected for testing were 5000 μg/mL for 4 h –S9, 4000 μg/mL for 4 h +S9, and 2000 μg/mL for 24 h –S9; testing ranged down to 500 μg/mL for all test conditions. At the end of the culture period, cells were analyzed for cytotoxicity and micronucleus induction by flow cytometry (Avlasevich et al., 2006; Bryce et al., 2007) using the In Vitro MicroFlow™ kit (Litron Laboratories, Rochester, NY) according to manufacturer’s instructions. Unless limited by cytotoxicity, 20,000 cells from each sample were analyzed for the frequency of micronuclei (MN) using a FACSCalibur™ dual-laser bench top system (Becton Dickinson Biosciences, San Jose, CA). Viable cell counts were determined and used to calculate relative increase in cell count (RICC) in treated cultures.
2.4. Chromosomal aberration assays
Chromosomal aberration assays using the CHO-WBL cell line were conducted as described previously (Hobbs et al., 2012) with slight modifications to meet current OECD test guideline 473 (OECD, 2014b) recommendations. AGIQ was formulated in sterile water and isoquercitrin was formulated in DMSO; formulations were administered at 1% and 5% of the final culture volume for isoquercitrin and AGIQ, respectively. Based on results of a preliminary toxicity test (Supplemental Data Table S5), 5000 μg/mL was selected as the top concentration of AGIQ for testing to meet the limit dose recommendation for non-cytotoxic chemicals. A sharp decline in cell growth was observed in preliminary toxicity experiments of isoquercitrin (Supplemental Data Table S6). Therefore, concentrations of 2000, 1750, 1500, 1250, 500 and 200 μg/mL isoquercitrin were selected for chemical treatment for 4 h with and without metabolic activation, and concentrations of 1000, 750, 500, 200, 100 and 50 μg/mL were selected for treatment for 20 h in the absence of metabolic activation. In separate experiments, duplicate cultures of exponentially growing cells seeded approximately 23 h earlier at 0.2 × 106 cells/mL in T25 flasks were exposed to various concentrations of AGIQ, isoquercitrin, vehicle or positive control chemical; exposure durations were 4 and 20–21 h in the absence of metabolic activation, and 4 h in the presence of metabolic activation. The composition of the S9 mix was as described above; the final concentration of S9 in the cultures was 2%. Mitomycin C and cyclophosphamide were used as the positive controls for cultures incubated in the absence and presence of S9, respectively. For each test condition, the frequency of chromosomal aberrations and mitotic index (percentage of metaphase cells) were determined at the three highest chemical concentrations or at 3–4 concentration levels corresponding to minimal, mild and moderate cytotoxicity. The mitotic index was determined from 1000 cells per replicate culture. Structural and numerical chromosome damage (e.g., chromatid and chromosome gaps and breaks, double minute, dicentric and ring chromosomes and other complex rearrangements) was determined from scoring 150 metaphase spreads per replicate culture on coded slides at 1000× magnification. The number of chromosome aberrations (with and without gaps) per cell, and the number of cells with structural damage (no gaps), were tabulated. If 20 metaphase cells with structural chromosomal damage (excluding gaps) were detected out of the first 50 cells scored, scoring for that culture was terminated. To assess numerical abnormalities, the number of polyploidy mitoses, including those with endoreduplicated chromosomes, per 150 metaphase spreads/replicate multiplied by 100 was determined (polyploidy index, PI). Viable cell counts were determined and used to calculate RICC in treated cultures.
2.5. Animal husbandry
Male and female B6C3F1 mice (Charles River Laboratories International, Inc.) and Sprague Dawley rats (Harlan Laboratories) were housed in polycarbonate cages with absorbent hardwood bedding in an AAALAC-accredited specific pathogen free facility with a 12-h light/12-h dark cycle and provided Certified Purina Pico Chow No. 5002 (Ralston Purina Co., St. Louis, MO) and water ad libitum. Male CD2-LacZ80/HazfBR mice (Muta™ Mouse; Japan Laboratory Animals, Inc.) were individually housed in econ or zyfone cages with bedding (ALPHA-dri™; Shepherd Specialty Papers) in a barrier facility with a 12-h light/12-h dark cycle and provided free access to irradiation-sterilized powder diet (CRF-1; Oriental Yeast) and water. All animals were 7–10 weeks of age at the time of treatment. The MN/comet studies were approved by the ILS, Inc. Institutional Animal Care and Use Committee, and procedures completed in compliance with the Animal Welfare Act Regulations, 9 CFR 1–4, and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 2011). The transgenic mouse studies were approved by the Institutional Animal Care and Use Committee of the BSRC and conducted in compliance with the “Act on Welfare and Management of Animals” (Act No. 105 of October 1, 1973; recent revision: Act No. 38 of June 12, 2013), “Standards Relating to the Care and Management of Laboratory Animals and Relief of Pain” (Ministry of the Environment Notification No. 88, April 28, 2006; recent revision: Ministry of the Environment Notification No. 84, August 30, 2013) and “Law for Securing Multiplicity of Living Organisms under the Use Control of Genetically-engineered Living Organisms” (Act No. 97 of June 18, 2003, Latest revision: Act No. 67 of June 13, 2014).
2.6. In vivo MN/comet assay experimental design
In vivo MN and comet assays were conducted according to OECD test guidelines 474 and 489 (OECD, 2014c, d; 2016). Based on the results of dose range finding studies, male and female B6C3F1 mice and Sprague Dawley rats (5 animals/dose group) were administered AGIQ or isoquercitrin at 2000 (limit dose), 1500 or 1000 mg/kg/day, vehicle (deionized water for AGIQ or corn oil for isoquercitrin), or the positive control compound, ethyl methanesulfonate (EMS; Sigma-Aldrich, St. Louis, MO) in 0.9% saline (Ricca Chemical Company, Arlington, TX) at 200 (mice) or 150 (rats) mg/kg/day, daily for three days by oral gavage. Three hours after the final dose, peripheral blood was collected for flow cytometric analysis of MN, and liver, duodenum and glandular stomach tissues were collected and single cell suspensions prepared as described previously (Hobbs et al., 2015); cell samples were frozen in liquid N2, and stored at −80 °C until analysis by the comet assay (Recio et al., 2012).
2.7. Erythrocyte micronucleus assay
Peripheral blood samples were processed for flow cytometric evaluation of micronucleated reticulocytes (MN-RET) as described previously (Witt et al., 2008). Cells were fixed and labeled using a MicroFlowPLUS Kit (Litron Laboratories, Rochester, NY) according to manufacturer’s directions and analyzed using a FACSCalibur flow cytometer (Becton Dickinson, Sunnyvale, CA). For each peripheral blood sample, 20,000 RET were analyzed to determine the frequency of MN-RET. More than 106 mature normochromatic erythrocytes were typically enumerated concurrently during MN-RET analysis, and the percentage of RET (%RET) among total erythrocytes was calculated as a measure of bone marrow toxicity.
2.8. Comet assay
Slides were prepared as described previously (Hobbs et al., 2015) and stored at room temperature in a desiccator until stained and scored. After staining slides with SYBR Gold™ (Molecular Probes, Invitrogen, Carlsbad, CA), 150 cells were scored per sample at 200× total magnification without scorers’ knowledge of animal identity using Comet Assay IV Imaging Software, Version 4.3.1 (Perceptive Instruments, Ltd., Suffolk, UK). The extent of DNA migration was characterized using the % tail DNA endpoint measurement (intensity of all tail pixels divided by the total intensity of all pixels in the comet, expressed as a percentage).
2.9. Muta™ Mouse mutation assay
Based on the results of dose range finding studies, male transgenic mice (Muta™ Mouse; 5 evaluated animals/dose group) were fed diet containing AGIQ or isoquercitrin at 5%, 1.5%, 0.5% or 0% for 28 days and tissues harvested three days following the final dose (Day 31). Additional groups (3 evaluated animals/group) were administered the positive control compound, N-ethyl-N-nitrosourea (ENU; Toronto Research Chemicals, Inc., Toronto, Canada), intraperitoneally at 100 mg/kg in sodium phosphate buffer (pH 6) once daily for two consecutive days and tissues harvested 10 days following the final dosing. Food weights were measured on Days 1, 8, 15, 22 and 29 and the mean daily food consumption (g/day) and the test substance intake (mg/kg/day) were calculated. Following euthanasia by inhalation of carbon dioxide, the liver, kidney, stomach and testis or liver and testis were removed from the animals for the AGIQ and isoquercitrin studies, respectively; tissues were observed macroscopically and stored in a −80 °C freezer.
Each frozen tissue sample was homogenized in freshly prepared homogenization buffer (11 mM Na2HPO4, 1.6 mM KH2PO4, 0.12 mM NaCl, 2.37 mM KCl, 0.2 M sucrose, 0.05 M EDTA, pH 8.0) containing 0.2 mg/mL RNase using a Dounce-type homogenizer prechilled on ice, gently layered onto Dounce buffer (12 mM Na2HPO4, 1.8 mM KH2PO4, 0.137 M NaCl, 2.7 mM KCl, 0.01 M EDTA, pH 8.0) containing 0.5 M sucrose in an ice-cooled centrifuge tube, and centrifuged at 3000 rpm (1710 g) for 10 min. The supernatant was removed and cold Dounce buffer containing 0.2 mg/mL RNase was added to the tube and mixed well. An equal volume of freshly prepared 2 mg/mL Proteinase K (Wako Pure Chemical Industries, Osaka, Japan) solution (containing 2% SDS and 0.1 M EDTA, pH 7.5) was added to the nuclei suspension and gently mixed by inversion. This suspension was incubated at 50 °C for 2–3 h until it became clear. An equal volume of fresh TE-saturated phenol/chloroform (1:1) was added to the solution and mixed by inversion several times, subsequently mixed by using a rotator for 10 min, and then centrifuged at 2500 rpm (1190 g) for 10 min. The upper aqueous layer was gently collected, transferred to another centrifuge tube, and reextracted with phenol/chloroform and then chloroform/isoamyl alcohol (24:1) as before. The aqueous layer was transferred to another centrifuge tube and genomic DNA was precipitated by gradually adding ethanol to the tube. Precipitated genomic DNA was transferred to a microtube containing 70% ethanol, incubated for 10 min and the contents centrifuged at 13,000 rpm (13,240 g) for 10 min. The supernatant was removed and residual ethanol allowed to evaporate at room temperature. Pelleted DNA was resuspended in TE buffer overnight at room temperature and stored in a refrigerator. DNA concentrations were adjusted to 100–600 μg/mL with TE buffer. Transpack packaging extract (Stratagene, La Jolla, CA) was used to package genomic DNA according to manufacturer’s instructions. The packaged DNA sample was stored on ice until use.
LB broth containing 2 mg/mL maltose in a baffled Erlenmeyer flask was inoculated (1/100) with a fresh overnight culture of Escherichia coli C (lacZ–, gal E–; cultured in the presence of 2 mg/mL maltose, 0.05 mg/mL ampicillin and 0.02 mg/mL kanamycin) and incubated for about 2–6 h at 37 °C with shaking at 120 strokes/min. Then the bacterial suspension was centrifuged at 1000 rpm for 10 min, the supernatant removed, and the cells suspended in LB broth containing 10 mM magnesium sulfate. The entire volume of packaged DNA was added to a tube containing 2 mL of the fresh E. coli suspension and incubated at room temperature for 30 min to allow the phage infection of bacteria to occur. Thirty microliters of a 10-fold dilution of infected bacteria in LB broth containing 10 mM magnesium sulfate were transferred to a tube containing 1 mL of the fresh E. coli suspension; LB top agar containing 10 mM (isoquercitrin) or 20 mM (AGIQ) magnesium sulfate was added to the tube and the contents were poured over a 150 mm LB agar plate for titer determination. Freshly made top agar containing 3 mg/mL (isoquercitrin) or 6 mg/mL (AGIQ) phenyl β-d-galactoside (Sigma-Aldrich, St. Louis, MO) was added to the tube containing bacteriophage-infected E. coli and the contents were poured over a 150 mm LB agar plate for mutant plaque selection. Agar plates were incubated at 37 °C overnight. The lacZ mutant frequency was calculated by dividing the number of mutant plaques by the total number of plaques. EDTA, TE-saturated phenol, and RNase were purchased from Nippon Gene (Toyama, Japan).
2.10. Data analyses
Statistical analysis of the data was performed using the Statistical Analysis System version 9.2 or 9.4 (SAS Institute, Cary, NC). For in vitro MN and in vivo MN/comet assays, homogeneity and normality of the vehicle control data were assessed using Levene’s test and the Shapiro-Wilk test, respectively. Homogeneous, normally distributed data were analyzed using one way analysis of variance (ANOVA) and treatment groups compared to the appropriate control group using Dunnett’s multiple comparison test. Dose-dependent changes were evaluated using linear regression. Data that were not homogeneous and normally distributed were transformed and re-assessed. For each assay, a one-tailed t-test was used to verify a positive response to the positive control compound (p < 0.05). For chromosomal aberration assays, mitotic indexes were analyzed for a decrease over vehicle controls using a one-tailed pairwise Student’s t-test and for linear trend with linear regression analysis. Percent structurally damaged cells (without gaps) and polyploidy indexes were analyzed for an increase over vehicle controls using a one-tailed pairwise Fisher’s Exact test and for linear trend using a one-tailed Cochran-Armitage test. For the transgenic mouse mutation studies, mutant frequency, food consumption, body weight, body weight gain, organ weight and relative organ weight data were tested by Bartlett’s test for homogeneity of variance. Dunnett’s multiple comparison test and Steel’s test were used for homogeneous and non-homogeneous data, respectively, to assess the statistical significance of differences between the negative control group and each test substance-treated group. The mutant frequency data for the negative control group and the positive control group were first tested by an F test, then by Student’s t-test or Aspin-Welch’s t-test when the F test was not significant, or significant, respectively.
For the bacterial mutation assays, a reproducible or dose-dependent 2-fold or greater increase in the number of revertant colonies relative to the vehicle control was considered to be a positive response. Criteria for a positive result in the in vitro and in vivo micronucleus assays and in vivo comet assays were at least one statistically significant dose group (p < 0.05), a dose group falling outside the range of laboratory historical control data, and a statistically significant trend test (p < 0.05). A test was considered equivocal if only one or two of these conditions were met (OECD, 2014a, c, d). In the case of chromosomal aberrations, a statistically significant increase for two or more concentrations in the absence of a significant trend test was considered a positive result (OECD, 2014b). For the transgenic mouse mutation assays, a mutant frequency in a test substance-treated group that was significantly different from that in the negative control group was considered to be a positive response (OECD, 2011).
3. Results
3.1. Bacterial reverse mutation assays
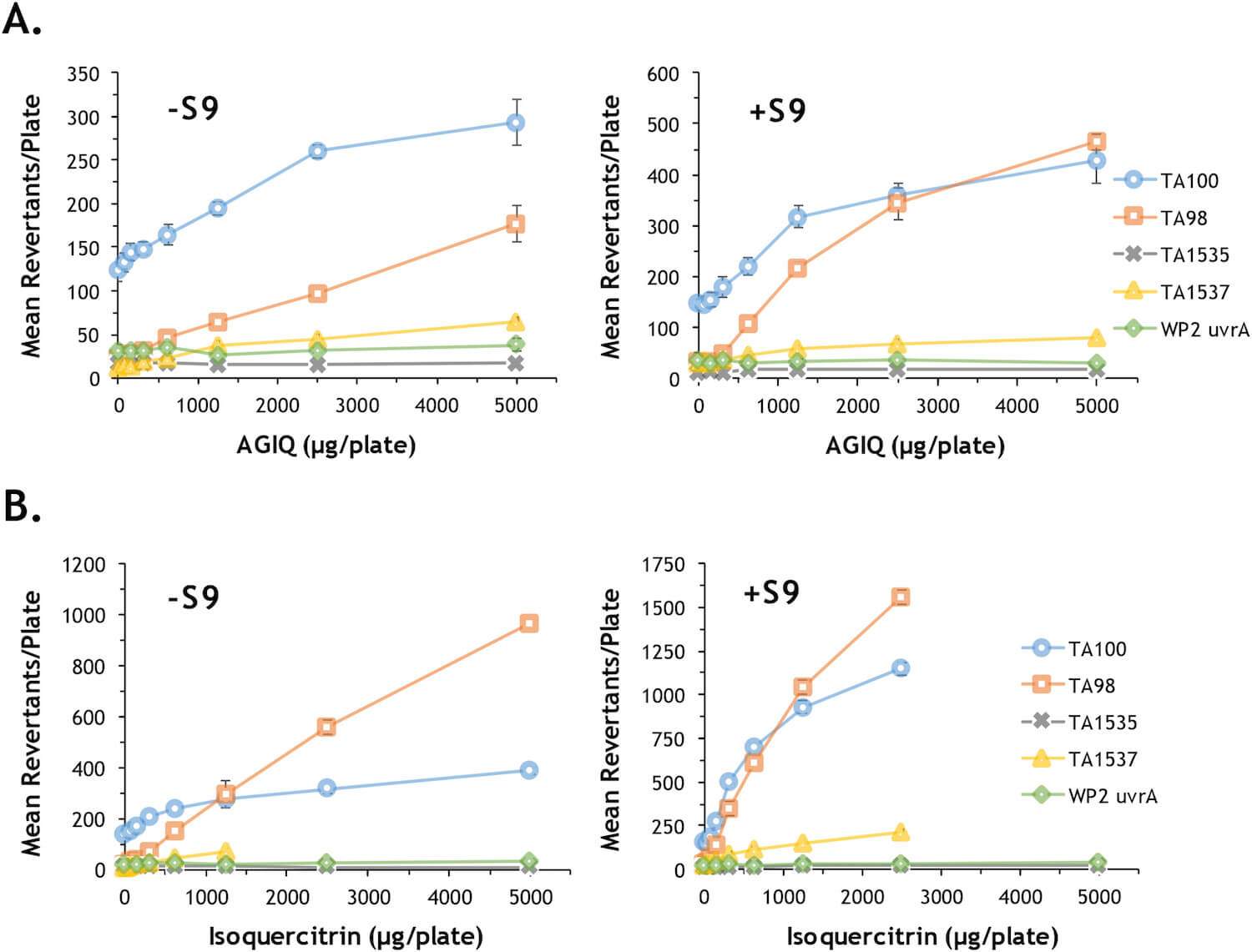
Isoquercitrin and AGIQ were tested up to a maximum of 5000 μg/plate, the upper limit specified by the OECD test guideline (OECD, 1997). Exposure to both chemicals induced 2-fold or greater increases in the number of revertant colonies relative to the vehicle control group in the TA100, TA98 and TA1537 strains without metabolic activation, and in the TA100, TA98, TA1537 and TA1535 (isoquercitrin only) strains in the presence of metabolic activation; the increases in revertants were dose-dependent (Fig. 2). The positive control chemicals induced positive responses in all strains, with or without metabolic activation (Supplemental Data Tables S7 and S8), and the mean number of revertant colonies in the controls were within the reference ranges calculated from the laboratory historical data. Similar results were obtained in preliminary dose finder studies (Supplemental Data Tables S1 and S2), demonstrating the reproducibility of the positive responses.
Fig. 2. Bacterial reverse mutation assays of AGIQ and isoquercitrin. Evaluation of the potential of AGIQ (A) and isoquercitrin (B) to induce mutations (revertant colonies) in the presence (+S9) and absence (–S9) of metabolic activation using five tester strains of bacteria. The data values, including results for positive control chemicals, are provided in Supplemental Data Tables S7 and S8.
3.2. In vitro MN assays
MN are identified by flow cytometry using a combination of characteristics of size (as measured by light scatter) and fluorescence (based on differential staining) that differentiates debris and necrotic and apoptotic cells from healthy cells containing micronuclei (Avlasevich et al., 2006; Bryce et al., 2007). MN frequency and cell viability data for cultures exposed to AGIQ are summarized in Table 1. After approximately 4 h in the presence and absence of metabolic activation, maximum cytotoxicity was within the recommended OECD guideline level (≤60%) at all concentrations. Measured MN frequencies at all concentrations were not statistically significantly elevated over the vehicle control. Exposure to cyclophosphamide, used as a positive control chemical requiring metabolic activation, and vinblastine, a positive control for a direct-acting response, resulted in statistically significant 4.5- and 17.9-fold inductions of micronuclei, respectively, as compared to concurrent vehicle controls. Following exposure to AGIQ for 24 h in the absence of metabolic activation, measured micronucleus frequencies at concentrations up to and including one inducing slightly greater than 60% cytotoxicity were not statistically increased over the vehicle control and fell within the laboratory historical vehicle control range. Treatment with vinblastine led to a statistically significant 14.3-fold induction of MN as compared to the vehicle control. Based on these results, the response to AGIQ was considered negative for all test conditions.
Table 1. Micronucleus assay results in TK6 cells exposed to AGIQ.
| Dose (μg/mL) | Micronucleus Frequency (%) | Apoptotic/Necrotic Cells (%) | RICC (%) | ||
|---|---|---|---|---|---|
| Mean | Fold Change | Mean | Fold Change | Mean | |
| 4 Hour Exposure with S9 | |||||
| DMSO | 0.59 | – | 1.8 | – | 100.0 |
| 0 | 0.53 | – | 1.4 | – | 100.0 |
| 156 | 0.51 | 1.0 | 1.5 | 1.1 | 76.3 |
| 313 | 0.47 | 0.9 | 1.5 | 1.0 | 96.3 |
| 625 | 0.56 | 1.1 | 1.8 | 1.3 | 112.6 |
| 1250 | 0.52 | 1.0 | 1.5 | 1.0 | 106.1 |
| 2500 | 0.61 | 1.2 | 1.6 | 1.1 | 100.8 |
| 5000 | 0.65 | 1.2 | 1.6 | 1.2 | 81.5 |
| CP (3) | 2.68a | 4.5 | 5.4 | 3.0 | 13.3 |
| 4 Hour Exposure without S9 | |||||
| DMSO | 0.46 | – | 1.6 | – | 100.0 |
| 0 | 0.44 | – | 1.4 | – | 100.0 |
| 156 | 0.42 | 1.0 | 1.5 | 1.0 | 47.3 |
| 313 | 0.36 | 0.8 | 1.8 | 1.2 | 83.0 |
| 625 | 0.50 | 1.1 | 1.5 | 1.0 | 83.0 |
| 1250 | 0.51 | 1.2 | 1.4 | 1.0 | 84.2 |
| 2500 | 0.55 | 1.3 | 1.6 | 1.1 | 61.7 |
| 5000 | 0.46 | 1.0 | 1.7 | 1.2 | 45.7 |
| VIN (0.005) | 8.22a | 17.9 | 7.7 | 4.7 | 11.2 |
| 24 Hour Exposure without S9 | |||||
| DMSO | 0.54 | – | 1.2 | – | 100.0 |
| 0 | 0.56 | – | 2.0 | – | 100.0 |
| 156 | 0.59 | 1.1 | 2.4 | 1.2 | 93.1 |
| 313 | 0.58 | 1.0 | 3.0 | 1.5 | 76.4 |
| 625 | 0.84 | 1.5 | 2.1 | 1.1 | 52.8 |
| 1250 | 0.77b | 1.4 | 2.0 | 1.0 | 35.6 |
| 2500 | 1.23c | 2.2 | 1.9 | 1.0 | 34.4 |
| 5000 | 1.51c | 2.7 | 1.7 | 0.9 | 34.4 |
| VIN (0.00075) | 7.73a | 14.3 | 8.7 | 7.3 | 13.8 |
RICC = relative increase in cell count; CP = cyclophosphamide; VIN = vinblastine.
- a Statistically positive at p < 0.05.
- b Statistically positive trend at p < 0.05.
- c Not analyzed due to excessive cytotoxicity.
The results of exposing TK6 cells to isoquercitrin are summarized in Table 2. Four hours in the presence of metabolic activation resulted in cytotoxicity >60% at the top two doses of 4000 and 3000 μg/mL; responses at these doses were potentially confounded by excessive cytotoxicity. Because the micronucleus data were considered non-homogeneous (Levene’s Test for homogeneity p-value = 0.0465), the data were transformed using the inverse function. Micronucleus frequencies at all concentrations, including the lowest cytotoxic concentration, were not significantly elevated over the vehicle control. Therefore, the response with metabolic activation was considered negative. The positive control, cyclophosphamide, induced a statistically positive 3-fold response. For TK6 cells exposed to isoquercitrin for 4 h in the absence of metabolic activation, cytotoxicity was much greater than 60% at the highest concentration tested (5000 μg/mL); excessive cytotoxicity and precipitate precluded flow cytometric evaluation at this concentration. The MN frequencies at the two highest concentrations evaluated were statistically elevated compared to the vehicle control and a dose response was measured (p < 0.05); however, the MN frequency values fell within the laboratory’s historical control range and the response was considered not to be biologically relevant. The positive control, vinblastine, induced a positive 11.8-fold response. Exposure of TK6 cells to isoquercitrin for 24 h in the absence of metabolic activation resulted in >60% cytotoxicity at the top three concentrations. Micronucleus data were analyzed up to and including one cytotoxic concentration (1000 mg/mL); MN frequency was statistically elevated over the vehicle control group and fell outside of the laboratory historical vehicle control range only at this cytotoxic concentration. In addition, MN frequency was not significantly elevated at the next lower dose (750 mg/mL) that induced a moderate level of cytotoxicity (57%). The positive control, vinblastine, induced a robust 11.6-fold increase in MN formation. Based on these results, the response to isoquercitrin exposure was considered to be negative under all conditions tested.
Table 2. Micronucleus assay results in TK6 cells exposed to isoquercitrin.
| Dose (μg/mL) | Micronucleus Frequency (%) | Apoptotic/Necrotic Cells (%) | RICC (%) | ||
|---|---|---|---|---|---|
| Mean | Fold Change | Mean | Fold Change | Mean | |
| 4 Hour Exposure with S9 | |||||
| DMSO | 0.88 | – | 2.2 | – | 100.0 |
| 500 | 0.84 | 1.0 | 2.1 | 0.9 | 125.9 |
| 1000 | 0.92 | 1.0 | 2.5 | 1.1 | 101.1 |
| 2000 | 1.06 | 1.2 | 2.9 | 1.3 | 74.9 |
| 3000 | 1.26 | 1.4 | 3.3 | 1.5 | −2.9 |
| 4000 | 1.29a | 1.5 | 3.3 | 1.5 | −22.2 |
| CP (3) | 2.68b | 3.0 | 5.8 | 2.6 | 37.9 |
| 4 Hour Exposure without S9 | |||||
| DMSO | 0.62 | – | 2.0 | – | 100.0 |
| 500 | 0.60 | 1.0 | 1.4 | 0.7 | 65.8 |
| 1000 | 0.86 | 1.4 | 2.0 | 1.0 | 72.5 |
| 1500 | 1.03b | 1.7 | 2.0 | 1.0 | 71.6 |
| 2500 | 1.08a,b | 1.7 | 2.6 | 1.3 | 56.6 |
| 5000 | NA | – | NA | – | NA |
| VIN (0.005) | 7.29b | 11.8 | 7.9 | 4.0 | 18.8 |
| 24 Hour Exposure without S9 | |||||
| DMSO | 0.74 | – | 1.5 | – | 100.0 |
| 500 | 0.87 | 1.2 | 1.7 | 1.1 | 64.4 |
| 750 | 1.00 | 1.4 | 1.9 | 1.3 | 43.0 |
| 1000 | 1.22b | 1.7 | 1.9 | 1.3 | 31.5 |
| 1500 | 1.22c | 1.7 | 2.3 | 1.6 | 30.5 |
| 2000 | 1.72c | 2.3 | 2.5 | 1.7 | 17.2 |
| VIN (0.00075) | 8.62b | 11.7 | 8.9 | 6.0 | 7.2 |
RICC = relative increase in cell count; CP = cyclophosphamide; VIN = vinblastine.
NA = sample not analyzable due to very heavy precipitate.
- a Significant trend at p < 0.05.
- b Significant at p < 0.05.
- c Not statistically analyzed due to excessive cytotoxicity.
3.3. In vitro chromosomal aberration assays
Studies were conducted to assess the potential for AGIQ or isoquercitrin to cause chromosomal aberrations in CHO–WBL Chinese hamster ovary cells. The results of the chromosomal aberration assays are summarized in Tables 3 and 4. For both studies, the percentage of vehicle control cells with structural chromosomal aberrations (excluding gaps) and numerical chromosomal damage observed as polyploidy did not exceed 5% and 10%, respectively, meeting requirements for a valid test. The positive control chemicals, mitomycin C and cyclophosphamide, induced statistically significant increases in the percentage of metaphase cells with at least one chromosomal aberration (excluding gaps) under all exposure conditions. AGIQ was minimally to moderately cytotoxic at concentrations of 1250 μg/mL and above as measured by mitotic index and relative increase in cell concentration (RICC), with the exception of 1250 and 2500 μg/mL for 4 h –S9, that induced increases in mitotic index. Cytotoxicity did not preclude scoring up to the limit concentration of 5000 μg/mL for all exposure conditions. No evidence of increased structural chromosomal damage or polyploidy was measured at any tested concentration.
Table 3. Chromosome aberration assay results in CHO cells exposed to AGIQ.
| Dose (μg/mL) | Viable Cells (x106)/mL | Mitotic Index (%) | % Damaged Cells | Polyploidy Index (%) |
|---|---|---|---|---|
| Mean | Mean | Mean | Mean | |
| 4 Hour Exposure without S9 | ||||
| 0 | 1.43 | 4.0 | 0.0 | 0.7 |
| 1250 | 1.37 | 5.8a | 0.3 | 1.0 |
| 2500 | 1.22 | 5.0 | 0.0 | 0.7 |
| 5000 | 1.32 | 3.5 | 0.3 | 0.7 |
| MMC (0.15) | 1.01 | 3.3 | 5.3b | 1.0 |
| 4 Hour Exposure with S9 | ||||
| 0 | 1.48 | 5.7 | 0.0 | 0.7 |
| 1250 | 1.38 | 3.0a | 0.0 | 0.0 |
| 2500 | 1.17 | 1.7a | 0.0 | 0.3 |
| 5000 | 1.14 | 2.2a,c | 0.0 | 0.0 |
| CP (5) | 0.91 | 1.6 | 40.0b | 1.0 |
| 20 Hour Exposure without S9 | ||||
| 0 | 1.18 | 5.6 | 0.0 | 0.7 |
| 1250 | 0.90 | 2.0a | 0.0 | 0.3 |
| 2500 | 0.88 | 2.4a | 0.0 | 1.0 |
| 5000 | 0.74 | 1.5c | 0.7 | 0.0 |
| MMC (0.075) | 1.15 | 4.1 | 4.7b | 0.7 |
Mitotic Index (%) = percent of cells in metaphase.
% Damaged Cells = percent of metaphase cells with at least one structural chromosomal aberration, excluding gaps.
Polyploid Index = percent of polyploid metaphase cells, including endoreduplicated metaphase cells.
MMC = Mitomycin C.
CP = Cyclophosphamide.
- a Significant at p < 0.05 by a one-tailed independent samples t-test.
- b Significant at p < 0.05 by a Fisher’s exact test.
- c Significant trend at p < 0.05 by linear regression.
Table 4. Chromosomal aberration assay results in CHO cells exposed to isoquercitrin.
| Dose (μg/mL) | Viable Cells (x105)/mL | Mitotic Index (%) | % Damaged Cells | Polyploidy Index (%) |
|---|---|---|---|---|
| Mean | Mean | Mean | Mean | |
| 4 Hour Exposure without S9 | ||||
| 0 | 9.95 | 7.5 | 0.0 | 0.0 |
| 200 | 10.56 | 5.3 | 0.3 | 0.3 |
| 1250 | 6.83 | 5.4a | 1.7c | 1.0 |
| 2000 | 5.87 | 2.6a,b | 4.3c,d | 0.0 |
| MMC (0.15) | 9.72 | 4.7a | 17.5c | 0.7 |
| 4 Hour Exposure with S9 | ||||
| 0 | 7.45 | 6.3 | 0.0 | 1.0 |
| 200 | 6.40 | 5.7 | 0.0 | 0.3 |
| 1500 | 6.56 | 5.3 | 1.3 | 0.3 |
| 2000 | 6.45 | 1.6a,b | 2.0c,d | 0.3 |
| CP (5) | 5.56 | 2.6a | 40.0c | 0.0 |
| 20 Hour Exposure without S9 | ||||
| 0 | 14.08 | 5.7 | 0.0 | 0.3 |
| 50 | 11.58 | 4.3 | 0.0 | 0.7 |
| 200 | 13.66 | 4.4 | 0.0 | 1.0 |
| 500 | 9.21 | 3.9 | 0.0 | 1.0 |
| 1000 | 6.49 | 2.0b | 0.7d | 1.0 |
| MMC (0.075) | 12.2 | 2.5a | 40.0c | 0.0 |
Mitotic Index (%) = percent of cells in metaphase.
% Damaged Cells = percent of metaphase cells with at least one structural chromosomal aberration, excluding gaps.
Polyploid Index = percent of polyploid metaphase cells, including endoreduplicated metaphase cells.
MMC = Mitomycin C.
CP = Cyclophosphamide.
- a Significant at p < 0.05 by a one-tailed independent samples t-test.
- b Significant trend at p < 0.05 by linear regression.
- c Significant at p < 0.05 by a Fisher’s exact test.
- d Significant trend at p < 0.05 by a one-tailed Cochran Armitage trend test.
Isoquercitrin induced moderate cytotoxicity in cultured CHO-WBL cells at a concentration of 2000 μg/mL following exposure for 4 h ± S9 and at 1000 μg/mL following exposure for 20 h –S9. A dose-related increase in percent damaged cells was observed following 4 h exposures; statistically significant increases fell outside the laboratory historical vehicle control range at 1250 and 2000 μg/mL for the –S9 exposure and at 2000 μg/mL for the +S9 exposure. No increase in the percent of damaged cells was observed at any tested concentration following continuous exposure for 20 h –S9. Polyploidy was not significantly increased at any tested concentration.
3.4. In vivo MN/comet assays
Based on the results of preliminary dose setting studies, combined MN/comet assays were conducted in which male and female B6C3F1 mice were administered AGIQ or isoquercitrin, and male and female Sprague Dawley® rats were administered AGIQ, at 1000, 1500 and 2000 (test guideline limit dose) mg/kg/day for 3 consecutive days. No exposure-related clinical signs were noted during any of the studies with either chemical and there were no abnormal gross organ observations at necropsy. One male rat in the 1500 mg/kg/day dose group died on day 3 shortly after dose administration, apparently as a consequence of a gavage error. Male mice administered 1500 or 2000 mg/kg/day AGIQ lost statistically less body weight as compared to the concurrent vehicle control group (−0.005% and −0.004% versus −3.3%). No marked changes in body weights were measured in male mice administered isoquercitrin or female mice administered either chemical. Likewise, no effects were observed on body weight in male or female rats administered AGIQ. The positive reference chemical, ethyl methanesulfonate (EMS), did not induce effects on mean body weight in the mouse studies, but did lead to a statistically significant loss of weight in both male and female rats in the AGIQ study (data not shown).
Results of flow cytometric analysis of MN-RET and RET frequencies are summarized in Tables 5 and 6. Under the conditions used in the MN/comet studies, no increase in the frequency of MN-RET was observed for male or female mice administered isoquercitrin. There was a statistically significant increase in MN-RET frequency in male mice administered 2000 mg/kg/day AGIQ without an associated positive dose response. Conversely, a statistically significant dose dependent trend in MN induction was measured in female mice administered AGIQ without any statistically positive dose groups. Since all the dose groups for both male and female mice had MN-RET frequencies falling within laboratory historical data for vehicle controls, and given that the statistically significant responses were not reproducible between male and female mice, we do not consider these findings to be biologically relevant. Moreover, no induction of MN was detected in response to administration of AGIQ in either male or female rats. No decrease in the % RET was measured in mice administered either AGIQ or isoquercitrin, indicating a lack of bone marrow cytotoxicity at doses tested up to the limit dose of the assay. In contrast, there was a statistically significant decrease in % RET in male and female rats administered 1000 and 2000 mg/kg/day AGIQ, respectively, indicating some effect of exposure on the bone marrow. In previous toxicokinetic studies using rats, AGIQ and isoquercitrin were demonstrated to be rapidly bioavailable following oral gavage administration of doses lower than used in this study (Makino et al., 2009; Nyska et al., 2016). There were statistically significant increases in MN-RET in both male and female mice and rats administered EMS as a concurrent positive control in all studies.
Table 5. Micronucleus assay results in mice and rats administered AGIQ.
| Dose (mg/kg/day) | Mice | Rats | ||
|---|---|---|---|---|
| % RETa | MN-RET/1000a | % RETa | MN-RET/1000a | |
| Males | ||||
| 0 | 1.72 ± 0.10 | 2.36 ± 0.18 | 2.34 ± 0.10 | 0.60 ± 0.08 |
| 1000 | 1.65 ± 0.13 | 2.52 ± 0.09 | 1.69 ± 0.15c | 1.01 ± 0.11 |
| 1500b | 1.74 ± 0.13 | 2.65 ± 0.29 | 1.92 ± 0.04 | 0.75 ± 0.17 |
| 2000 | 1.63 ± 0.06 | 3.19 ± 0.28c | 1.95 ± 0.25 | 1.08 ± 0.20 |
| EMS | 0.92 ± 0.06 | 16.27 ± 1.87c | 1.02 ± 0.06 | 3.73 ± 0.46 |
| Females | ||||
| 0 | 1.54 ± 0.23 | 2.06 ± 0.21 | 1.29 ± 0.05 | 0.74 ± 0.06 |
| 1000 | 1.73 ± 0.25 | 2.56 ± 0.17 | 1.08 ± 0.09 | 0.86 ± 0.09 |
| 1500 | 1.68 ± 0.05 | 2.81 ± 0.21 | 1.05 ± 0.08 | 0.72 ± 0.13 |
| 2000 | 1.83 ± 0.09 | 2.80 ± 0.33d | 0.98 ± 0.11c | 0.65 ± 0.11 |
| EMS | 0.88 ± 0.09 | 25.49 ± 1.76c | 0.47 ± 0.08 | 3.76 ± 0.69 |
EMS = ethyl methanesulfonate administered at 150 (rats) or 200 (mice) mg/kg/day.
- a Group mean ± standard error of the mean.
- b One rat died due to a gavage error (n = 4).
- c Significant at p < 0.05.
- d Significant trend at p < 0.05.
Table 6. Micronucleus assay results in mice administered isoquercitrin.
| Dose (mg/kg/day) | % RETa | MN-RET/1000a |
|---|---|---|
| Males | ||
| 0 | 1.28 ± 0.01 | 2.92 ± 0.36 |
| 1000 | 1.54 ± 0.19 | 2.48 ± 0.19 |
| 1500 | 1.49 ± 0.08 | 2.33 ± 0.25 |
| 2000 | 1.44 ± 0.04 | 2.74 ± 0.13 |
| EMS | 0.84 ± 0.09 | 16.90 ± 2.35b |
| Females | ||
| 0 | 1.01 ± 0.15 | 1.93 ± 0.13 |
| 1000 | 1.37 ± 0.08 | 2.10 ± 0.17 |
| 1500 | 1.47 ± 0.12 | 2.19 ± 0.25 |
| 2000 | 1.36 ± 0.20c | 2.95 ± 0.26 |
| EMS | 0.50 ± 0.09 | 31.56 ± 2.21b |
EMS = ethyl methanesulfonate administered at 200 mg/kg/day.
- a Group mean ± standard error of the mean.
- b Significant at p < 0.05.
- c Significant trend at p < 0.05.
The results of the assessment of DNA damage in several potential target organs of mice and rats administered AGIQ or isoquercitrin, as measured by the comet assay, are provided in Tables 7 and 8. Under the conditions of the assay, there was no increase in DNA damage measured in liver, duodenum or stomach tissue in male or female mice administered AGIQ at any of the tested doses. There was a statistically positive dose dependent response in DNA damage measured in liver from female, but not male, mice; however, all DNA damage results were within ILS’ historical negative (vehicle) control range. Given that the equivocal response in female mice was not reproduced in male mice, and that all % tail DNA values fell within the range of historical vehicle control data, it is unlikely that the equivocal response in the comet assay is biologically relevant. This interpretation is further supported by the lack of DNA damage detected in liver, stomach or duodenum of both male and female rats administered AGIQ. In the isoquercitrin study, there was no increase in DNA damage measured in liver, stomach or duodenum of female mice, or in the liver of male mice, administered isoquercitrin compared to the concurrent control groups. Increases in DNA damage were detected in liver, stomach and duodenum of mice and rats administered EMS in all studies of AGIQ and isoquercitrin.
Table 7. Comet assay results in mice and rats administered AGIQ.
| Dose (mg/kg/day) | % Tail DNA in Micea | % Tail DNA in Ratsa | ||||
|---|---|---|---|---|---|---|
| Duodenum | Liver | Stomach | Duodenum | Liver | Stomach | |
| Males | ||||||
| 0 | 8.10 ± 1.70 | 3.87 ± 0.90 | 5.75 ± 1.36 | 6.06 ± 0.84 | 8.56 ± 1.42 | 7.05 ± 0.82 |
| 1000 | 6.92 ± 0.46 | 5.93 ± 1.46 | 7.22 ± 0.82 | 6.47 ± 1.08 | 7.81 ± 1.15 | 10.65 ± 1.91 |
| 1500b | 6.26 ± 0.53 | 6.56 ± 1.40 | 6.98 ± 1.55 | 7.50 ± 1.11 | 9.45 ± 1.82 | 10.02 ± 1.50 |
| 2000 | 6.72 ± 0.52 | 2.96 ± 0.60 | 6.18 ± 0.79 | 7.26 ± 0.79 | 9.44 ± 1.73 | 11.76 ± 1.42 |
| EMS | 19.01 ± 1.55c | 19.38 ± 2.13c | 22.68 ± 1.20c | 18.53 ± 1.28c | 24.62 ± 1.14c | 29.88 ± 1.37c |
| Females | ||||||
| 0 | 0.99 ± 0.19 | 3.04 ± 0.77 | 4.28 ± 0.88 | 10.14 ± 0.93 | 6.68 ± 0.68 | 7.93 ± 1.17 |
| 1000 | 1.70 ± 0.42 | 3.98 ± 0.38 | 6.45 ± 0.72 | 11.23 ± 0.56 | 7.62 ± 0.87 | 8.56 ± 0.69 |
| 1500 | 1.32 ± 0.26 | 4.67 ± 0.23 | 7.63 ± 1.95 | 10.25 ± 0.88 | 7.26 ± 0.69 | 9.84 ± 1.55 |
| 2000 | 1.69 ± 0.24 | 4.01 ± 0.22d | 8.05 ± 0.89 | 8.54 ± 0.49 | 8.42 ± 0.73 | 7.87 ± 1.38 |
| EMS | 11.88 ± 1.05c | 15.43 ± 1.07c | 24.95 ± 1.23c | 15.76 ± 1.89c | 27.18 ± 0.85c | 31.97 ± 3.05c |
EMS = ethyl methanesulfonate administered at 150 (rats) or 200 (mice) mg/kg/day.
- a Group mean ± standard error of the mean..
- b One rat died due to an oral gavage error (n = 4).
- c Significant at p < 0.05.
- d Significant trend at p < 0.05.
Table 8. Comet assay results in mice administered isoquercitrin.
| Dose (mg/kg/day) | % Tail DNAa | ||
|---|---|---|---|
| Duodenum | Liver | Stomach | |
| Males | |||
| 0 | 4.91 ± 1.99 | 3.74 ± 0.27 | 10.90 ± 2.82 |
| 1000 | 4.31 ± 0.95 | 3.38 ± 0.98 | 5.64 ± 0.90 |
| 1500 | 3.98 ± 0.93 | 3.42 ± 0.88 | 5.58 ± 1.80 |
| 2000 | 2.97 ± 0.61 | 3.01 ± 0.67 | 4.70 ± 0.69b |
| EMS | 13.95 ± 1.31c | 18.38 ± 1.61c | 25.82 ± 0.84c |
| Females | |||
| 0 | 2.24 ± 0.50 | 5.96 ± 0.42 | 3.85 ± 0.68 |
| 1000 | 1.54 ± 0.41 | 5.56 ± 0.40 | 3.74 ± 0.57 |
| 1500 | 1.85 ± 0.10 | 5.87 ± 0.38 | 6.86 ± 1.76 |
| 2000 | 1.71 ± 0.31 | 6.17 ± 0.50 | 6.51 ± 0.71 |
| EMS | 13.31 ± 0.58c | 21.28 ± 1.06c | 30.95 ± 2.03c |
EMS = ethyl methanesulfonate administered at 200 mg/kg/day.
- a Group mean ± standard error of the mean.
- b Dose dependent response at p < 0.05.
- c Significant at p < 0.05.
3.5. In vivo Muta™ Mouse mutation assays
For assessment of in vivo mutation, liver was selected for evaluation as a major site of xenobiotic metabolism, kidney as the possible target tissue of quercetin, an aglycone metabolite of the test substances (Makino et al., 2009), glandular stomach as the first site of exposure to the test substances and testis to evaluate the effect on germ cells. In all groups exposed to AGIQ, all mice produced unnaturally yellow urine; yellow urine was also observed in 1/6, 2/6 and 2/6 mice in the 0.5%, 1.5% and 5% isoquercitrin dose groups, respectively. Since the color of AGIQ and isoquercitrin is yellow, the enhanced yellow color of the urine was presumed to be due to excretion of the test substances or a derivative. No other changes in clinical signs, no apparent decreases of body weight, and no clear reduction of food consumption were observed in any of the dose groups for either chemical (data not shown). Mean intake amounts of AGIQ and isoquercitrin were 680, 2164 and 6895 mg/kg/day and 821, 2188 and 8575 mg/kg/day at 0.5, 1.5 and 5.0%, respectively. There were no differences in the organ weights or relative organ weights of AGIQ groups compared with those of the negative control group (data not shown; these endpoints were not assessed for isoquercitrin). During macroscopic observation, white patches were observed on the surface of the kidneys of one animal in the 0.5% AGIQ group; this observation was considered to be incidental.
The mutation frequency results are provided in Table 9. The total number of plaques obtained per animal was greater than the 125,000–300,000 minimum criterion recommended by the OECD test guideline (OECD, 2011), ranging from 0.7–2.6 × 106 and 0.4–1.3 × 106 for the AGIQ and isoquercitrin studies, respectively. No statistically significant increases were observed in any of the tissues from animals administered AGIQ or isoquercitrin at any of the tested doses compared with the concurrent negative control groups. Mutant frequencies were high in the kidneys of one of the negative control animals and one of the animals in the 0.5% group in the AGIQ study; likewise, mutant frequencies were high in the testis of another of the negative control animals and another of the animals in the 0.5% group. These increases were judged to be incidental because they were observed in only one mouse per group and there was no dose relationship.
Table 9. Results of Muta™ mouse mutation assays of AGIQ and isoquercitrin.
| Dose (w/w%) | Mutant frequency (×10−6)a | |||
|---|---|---|---|---|
| Liver | Kidney | Stomach | Testis | |
| alpha-Glycosyl isoquercitrin | ||||
| 0 | 52.6 ± 20.5 | 55.4 ± 31.3 | 42.1 ± 4.6 | 22.7 ± 12.6 |
| 0.5 | 34.4 ± 8.5 | 54.8 ± 33.5 | 44.2 ± 15.9 | 23.1 ± 16.9 |
| 1.5 | 44.0 ± 14.3 | 40.2 ± 6.6 | 43.6 ± 9.2 | 13.4 ± 3.0 |
| 5 | 41.1 ± 8.0 | 35.4 ± 4.0 | 41.1 ± 8.6 | 17.3 ± 1.1 |
| ENU | 124.3 ± 9.8b | 100.4 ± 12.9 | 441.1 ± 32.6c | 48.0 ± 8.6b |
| Isoquercitrin | ||||
| 0 | 61.8 ± 20.9 | – | – | 70.5 ± 12.2 |
| 0.5 | 62.0 ± 11.9 | – | – | 54.7 ± 16.1 |
| 1.5 | 73.5 ± 20.4 | – | – | 62.1 ± 15.2 |
| 5 | 70.1 ± 17.0 | – | – | 65.3 ± 16.5 |
| ENU | 222.9 ± 27.6b | – | – | 108.8 ± 14.6b |
ENU = N-Ethyl-N-nitrosourea administered at 100 mg/kg.
- a Group mean ± standard deviation.
- b Significant at p ≤ 0.05 by a Student’s t-test.
- c Significant at p ≤ 0.05 by an Aspin-Welch t-test.
Statistically significant increases in mutant frequencies were observed in the liver, stomach and testis of the positive control groups in the AGIQ study relative to the concurrent negative controls; although a statistical increase was not measured in the kidney of positive control animals, the mutant frequency increased nearly two-fold compared with the concurrent negative control group and was comparable to the laboratory historical positive control data (mean = 120.7 ± 39.4, n = 20). Mutant frequencies were also statistically increased in the liver and testis of the positive control groups in the isoquercitrin study relative to the concurrent negative controls.
4. Discussion
Quercetin, a natural flavonol ubiquitous in plants and present in some fruits, berries and onions at relatively high concentrations, has been reported to possess antioxidant, anti-carcinogenic, anti-inflammatory and cardioprotective properties (Erlund, 2004; Middleton et al., 2000). However, poor oral bioavailability and insolubility in water has limited its use as a dietary supplement or food additive. Conjugation of glucose moieties has been demonstrated to significantly enhance solubility and absorption of quercetin glycosides; in a Wistar rat study, bioavailability over 12 h was measured to be 2.0%, 12% and 35% for quercetin, isoquercitrin and AGIQ, respectively (Makino et al., 2009). Like quercetin, both isoquercitrin and AGIQ are reported to have properties potentially beneficial to human health, including anti-oxidant effects (Li et al., 2011; Nishimura et al., 2010; Shimada et al., 2010; Valentova et al., 2014), but offer the advantages of synthetic manufacture and greater utility as food additives while maintaining potential health benefits.
The anti-oxidant effects of quercetin have been closely linked to the potential generation of reactive pro-oxidant intermediates (Boots et al., 2003; Metodiewa et al., 1999); indeed, quercetin has been shown to exert a variety of mutagenic and genotoxicant effects in vitro(Engen et al., 2015; Utesch et al., 2008). However, for the most part, evidence of mutagenic or chromosome damaging properties related to quercetin exposure has not been provided by in vivo studies (Middleton et al., 2000; Utesch et al., 2008). There are very little published data investigating the genotoxic potential of isoquercitrin and AGIQ (Engen et al., 2015). In support of regulatory acceptance of expanded global marketing of products containing AGIQ, comprehensive GLP-compliant testing to ensure the safety of this chemical as a food additive has been ongoing in accordance with current OECD and EFSA test guidelines. We originally evaluated the genotoxicity and toxicity of CGTase, an enzyme derived from Bacillus pseudalcaliphilus DK-1139, used in the enzymatic production of AGIQ (Fig. 1); results supported its safe use (Maronpot et al., 2016). We recently reported the results of 90-day toxicity and toxicokinetic studies of AGIQ, establishing no observable adverse effect levels (NOAEL) of 5.0% in the diet for both male and female Sprague Dawley rats (3461 and 3867 mg/kg/day, respectively) (Nyska et al., 2016). The studies reported here (results summarized in Table 10) fill a critical information gap regarding potential genetic toxicity of isoquercitrin and AGIQ. Although both these chemicals induced mutations in several strains of bacteria, with and without metabolic activation, there was no evidence of mutagenic potential of either chemical detected in any of the multiple tissues examined in transgenic mice. Likewise, although isoquercitrin tested positive in the chromosomal aberration assay in CHO cells, results of all other in vitro and in vivo studies assessing numerical or structural chromosomal damage or DNA damage were negative for both chemicals. These results are consistent with the body of literature indicating a general lack of in vivo genotoxicity of flavonoids (Hobbs et al., 2015; Middleton et al., 2000; Utesch et al., 2008).
Table 10. Summary of genotoxicity assessments of AGIQ and isoquercitrin.
| Assay | alpha-Glycosyl Isoquercitrin | Isoquercitrin |
|---|---|---|
| Bacterial mutagenicity | positive | positive |
| in vitro micronucleus | negative | negativea |
| in vitro chromosome aberration | negative | positive |
| in vivo micronucleus | negative (mb, r) | negative |
| in vivo Comet | ||
| liver | negative (mc, r) | negative |
| duodenum | negative (m, r) | negative |
| stomach | negative (m, r) | negative |
| in vivo mutation (Muta™ Mouse) | negative | negative |
m = mice; r = rats.
- a Positive response (4 h –S9); however, group means fall within laboratory historical negative control data.
- b Positive dose group in males and positive trend in females; however, group means fall within laboratory historical negative control data.
- c Positive trend in females; however, group means fall within laboratory historical negative control data.
5. Conclusions
Large quantities of isoquercitrin and its more water soluble and bioavailable glycosylated derivative, AGIQ, can be manufactured enzymatically to support widespread use as a food ingredient source of flavonol possessing purported antioxidant benefits. The results of the comprehensive set of studies reported here demonstrate a lack of in vivo genotoxicity of AGIQ, supplementing existing toxicity data that support the safe use of AGIQ in food and beverages at estimated levels of dietary exposures.
Acknowledgements
This work was conducted at ILS, Inc. and the BSRC and funded by San-Ei Gen, FFI, a manufacturer of AGIQ. Maronpot Consulting LLC is a consultant for ILS, Inc. and San-Ei Gen, F.F.I., Inc. ILS, Inc. and the BSRC were responsible for the study design, the collection, analysis, and interpretation of data, and the writing of the final study reports and manuscript. The decision to submit the paper for publication was made by San-Ei Gen, F.F.I., Inc. The authors acknowledge the contributions of staff members at ILS and BSRC who provided technical, animal care, formulation, dosing, necropsy, analytical chemistry, and quality assurance services in support of these studies.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.fct.2017.12.059.
Transparency document
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.fct.2017.12.059.
References
Amado et al., 2009
N.G. Amado, D.M. Cerqueira, F.S. Menezes, J.F. da Silva, V.M. Neto, J.G. Abreu
Isoquercitrin isolated from Hyptis fasciculata reduces glioblastoma cell proliferation and changes beta-catenin cellular localization
Anti Canc. Drugs, 20 (2009), pp. 543-552
Ames et al., 1975
B.N. Ames, J. McCann, E. Yamasaki
Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test
Mutat. Res., 31 (1975), pp. 347-364
Avlasevich et al., 2006
S.L. Avlasevich, S.M. Bryce, S.E. Cairns, S.D. Dertinger
In vitro micronucleus scoring by flow cytometry: differential staining of micronuclei versus apoptotic and necrotic chromatin enhances assay reliability
Environ. Mol. Mutagen., 47 (2006), pp. 56-66
Boots et al., 2003
A.W. Boots, N. Kubben, G.R. Haenen, A. Bast
Oxidized quercetin reacts with thiols rather than with ascorbate: implication for quercetin supplementation
Biochem. Biophys. Res. Commun., 308 (2003), pp. 560-565
Bryce et al., 2007
S.M. Bryce, J.C. Bemis, S.L. Avlasevich, S.D. Dertinger
In vitro micronucleus assay scored by flow cytometry provides a comprehensive evaluation of cytogenetic damage and cytotoxicity
Mutat. Res., 630 (2007), pp. 78-91
Edenharder et al., 2003
R. Edenharder, H. Krieg, V. Kottgen, K.L. Platt
Inhibition of clastogenicity of benzo[a]pyrene and of its trans-7,8-dihydrodiol in mice in vivo by fruits, vegetables, and flavonoids
Mutat. Res., 537 (2003), pp. 169-181
EFSA, 2010
EFSA
Guidance on the data required for the risk assessment of flavourings to be used in or on foods
European Food Safety Authority, Parma, Italy
EFSA J., 8 (2010), p. 1623
EFSA, 2011
EFSA
Scientific opinion on genotoxicity testing strategies applicable to food and feed safety assessment
EFSA J., 9 (2011), p. 2379
Engen et al., 2015
A. Engen, J. Maeda, D.E. Wozniak, C.A. Brents, J.J. Bell, M. Uesaka, Y. Aizawa, T.A. Kato
Induction of cytotoxic and genotoxic responses by natural and novel quercetin glycosides
Mutat. Res., 784–785 (2015), pp. 15-22
Erlund, 2004
I. Erlund
Review of the flavonoids quercetin, hesperetin and naringenin. Dietary sources, bioactivities, and epidemiology
Nutr. Res., 24 (2004), pp. 851-874
FDA, 2000a
FDA
U.S. Food and Drug Administration, Office of Food Additive Safety. Redbook 2000: Toxicological Principles for the Safety Assessment of Food Ingredients. IV.C.1.b. In Vitro Mammalian Chromosomal Aberration Test
(2000)
FDA, 2000b
FDA
U.S. Food and Drug Administration, Office of Food Additive Safety. Redbook 2000: Toxicological Principles for the Safety Assessment of Food Ingredients. IV.C.1.d. Mammalian Erythrocyte Micronucleus Test
(2000)
FDA, 2000c
FDA
U.S. Food and Drug Administration, Office of Food Additive Safety. Redbook 2000: Toxicological Principles for the Safety Assessment of Food Ingredients. IV.C.1.a. Bacterial Reverse Mutation Test
(2000)
FDA, 2007
FDA
Agency Response Letter GRAS Notice No. GRN00220 [Alpha-glycosyl Isoquercitrin]
U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition
(2007)
Fujii et al., 2013
Y. Fujii, M. Kimura, Y. Ishii, R. Yamamoto, R. Morita, S.M. Hayashi, K. Suzuki, M. Shibutani
Effect of enzymatically modified isoquercitrin on preneoplastic liver cell lesions induced by thioacetamide promotion in a two-stage hepatocarcinogenesis model using rats
Toxicol, 305 (2013), pp. 30-40
Gasparotto Junior et al., 2011
A. Gasparotto Junior, F.M. Gasparotto, E.L. Lourenco, S. Crestani, M.E. Stefanello, M.J. Salvador, J.E. da Silva-Santos, M.C. Marques, C.A. Kassuya
Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: evidence for the inhibition of angiotensin converting enzyme
J. Ethnopharmacol., 134 (2011), pp. 363-372
Gatehouse et al., 1994
D. Gatehouse, S. Haworth, T. Cebula, E. Gocke, L. Kier, T. Matsushima, C. Melcion, T. Nohmi, T. Ohta, S. Venitt, et al.
Recommendations for the performance of bacterial mutation assays
Mutat. Res., 312 (1994), pp. 217-233
Hara et al., 2014
S. Hara, R. Morita, T. Ogawa, R. Segawa, N. Takimoto, K. Suzuki, N. Hamadate, S.M. Hayashi, A. Odachi, I. Ogiwara, S. Shibusawa, T. Yoshida, M. Shibutani
Tumor suppression effects of bilberry extracts and enzymatically modified isoquercitrin in early preneoplastic liver cell lesions induced by piperonyl butoxide promotion in a two-stage rat hepatocarcinogenesis model
Exp. Toxicol. Pathol., 66 (2014), pp. 225-234
Harwood et al., 2007
M. Harwood, B. Danielewska-Nikiel, J.F. Borzelleca, G.W. Flamm, G.M. Williams, T.C. Lines
A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties
Food Chem. Toxicol., 45 (2007), pp. 2179-2205
Hasumura et al., 2004
M. Hasumura, K. Yasuhara, T. Tamura, T. Imai, K. Mitsumori, M. Hirose
Evaluation of the toxicity of enzymatically decomposed rutin with 13-weeks dietary administration to Wistar rats
Food Chem. Toxicol., 42 (2004), pp. 439-444
Hobbs et al., 2012
C.A. Hobbs, C. Swartz, R. Maronpot, J. Davis, L. Recio, S.M. Hayashi
Evaluation of the genotoxicity of the food additive, gum ghatti
Food Chem. Toxicol., 50 (2012), pp. 854-860
Hobbs et al., 2015
C.A. Hobbs, C. Swartz, R. Maronpot, J. Davis, L. Recio, M. Koyanagi, S.M. Hayashi
Genotoxicity evaluation of the flavonoid, myricitrin, and its aglycone, myricetin
Food Chem. Toxicol., 83 (2015), pp. 283-292
Institute of Laboratory Animal Resources, 2011
Institute of Laboratory Animal Resources
Guide for the Care and Use of Laboratory Animals
the National Academies Press, Washington, DC (2011)
Kangawa et al., 2017a
Y. Kangawa, T. Yoshida, H. Abe, Y. Seto, T. Miyashita, M. Nakamura, T. Kihara, S.M. Hayashi, M. Shibutani
Anti-inflammatory effects of the selective phosphodiesterase 3 inhibitor, cilostazol, and antioxidants, enzymatically-modified isoquercitrin and alpha-lipoic acid, reduce dextran sulphate sodium-induced colorectal mucosal injury in mice
Exp. Toxicol. Pathol., 69 (2017), pp. 179-186
Kangawa et al., 2017b
Y. Kangawa, T. Yoshida, K. Maruyama, M. Okamoto, T. Kihara, M. Nakamura, M. Ochiai, Y. Hippo, S.M. Hayashi, M. Shibutani
Cilostazol and enzymatically modified isoquercitrin attenuate experimental colitis and colon cancer in mice by inhibiting cell proliferation and inflammation
Food Chem. Toxicol., 100 (2017), pp. 103-114
Kim et al., 2010
Y. Kim, S. Narayanan, K.O. Chang
Inhibition of influenza virus replication by plant-derived isoquercetin
Antivir. Res., 88 (2010), pp. 227-235
Kimura et al., 2013
M. Kimura, Y. Fujii, R. Yamamoto, A. Yafune, S.M. Hayashi, K. Suzuki, M. Shibutani
Involvement of multiple cell cycle aberrations in early preneoplastic liver cell lesions by tumor promotion with thioacetamide in a two-stage rat hepatocarcinogenesis model
Exp. Toxicol. Pathol., 65 (2013), pp. 979-988
Li et al., 2011
R. Li, C. Yuan, C. Dong, S. Shuang, M.M. Choi
In vivo antioxidative effect of isoquercitrin on cadmium-induced oxidative damage to mouse liver and kidney
N. Schmied. Arch. Pharmacol., 383 (2011), pp. 437-445
Makino et al., 2009
T. Makino, R. Shimizu, M. Kanemaru, Y. Suzuki, M. Moriwaki, H. Mizukami
Enzymatically modified isoquercitrin, alpha-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats
Biol. Pharm. Bull., 32 (2009), pp. 2034-2040
Manach et al., 1997
C. Manach, C. Morand, C. Demigne, O. Texier, F. Regerat, C. Remesy
Bioavailability of rutin and quercetin in rats
FEBS Lett., 409 (1997), pp. 12-16
Maron and Ames, 1983
D.M. Maron, B.N. Ames
Revised methods for the Salmonella mutagenicity test
Mutat. Res., 113 (1983), pp. 173-215
Maronpot et al., 2016
R.R. Maronpot, C.A. Hobbs, J. Davis, C. Swartz, M. Boyle, M. Koyanagi, S. Hayashi
Genetic and rat toxicity studies of cyclodextrin glucanotransferase
Toxicol. Rep, 3 (2016), pp. 381-392
Metodiewa et al., 1999
D. Metodiewa, A.K. Jaiswal, N. Cenas, E. Dickancaite, J. Segura-Aguilar
Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product
Free Radic. Biol. Med., 26 (1999), pp. 107-116
Middleton et al., 2000
E. Middleton Jr., C. Kandaswami, T.C. Theoharides
The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer
Pharmacol. Rev., 52 (2000), pp. 673-751
Morita et al., 2011
R. Morita, K. Shimamoto, Y. Ishii, K. Kuwata, B. Ogawa, M. Imaoka, S.M. Hayashi, K. Suzuki, M. Shibutani, K. Mitsumori
Suppressive effect of enzymatically modified isoquercitrin on phenobarbital-induced liver tumor promotion in rats
Arch. Toxicol., 85 (2011), pp. 1475-1484
Mortelmans and Zeiger, 2000
K. Mortelmans, E. Zeiger
The Ames Salmonella/microsome mutagenicity assay
Mutat. Res., 455 (2000), pp. 29-60
Nishimura et al., 2010
J. Nishimura, Y. Saegusa, Y. Dewa, M. Jin, M. Kawai, S. Kemmochi, T. Harada, S.M. Hayashi, M. Shibutani, K. Mitsumori
Antioxidant enzymatically modified isoquercitrin or melatonin supplementation reduces oxidative stress-mediated hepatocellular tumor promotion of oxfendazole in rats
Arch. Toxicol., 84 (2010), pp. 143-153
Nyska et al., 2016
A. Nyska, S.M. Hayashi, M. Koyanagi, J.P. Davis, M.P. Jokinen, Y. Ramot, R.R. Maronpot
Ninety-day toxicity and single-dose toxicokinetics study of alpha-glycosyl isoquercitrin in Sprague-Dawley rats
Food Chem. Toxicol., 97 (2016), pp. 354-366
OECD, 1997
OECD
OECD Guideline for the Testing of Chemicals: Bacterial Reverse Mutation Test
(1997)
471
OECD, 1998
OECD
OECD Guideline for the Testing of Chemicals: Repeated Dose 90-Day Oral Toxicity Study in Rodents
(1998)
408
OECD, 2010
OECD
OECD Guideline for the Testing of Chemicals: Toxicokinetics
(2010)
417
OECD, 2011
OECD
OECD Guideline for the Testing of Chemicals: Transgenic Rodent Somatic and Germ Cell Gene Mutation Assays
(2011)
488
OECD, 2014a
OECD
OECD Guideline for the Testing of Chemicals: in Vitro Mammalian Cell Micronucleus Test
(2014)
487
OECD, 2014b
OECD
OECD Guideline for the Testing of Chemicals: in Vitro Mammalian Chromosomal Aberration Test
(2014)
473
OECD, 2014c
OECD
OECD Guideline for the Testing of Chemicals: in Vivo Mammalian Alkaline Comet Assay
(2014)
489
OECD, 2014d
OECD
OECD Guideline for the Testing of Chemicals: Mammalian Erythrocyte Micronucleus Test
(2014)
474
OECD, 2016
OECD
OECD Guideline for the Testing of Chemicals: Mammalian Erythrocyte Micronucleus Test
(2016)
474
Recio et al., 2012
L. Recio, G.E. Kissling, C.A. Hobbs, K.L. Witt
Comparison of Comet assay dose-response for ethyl methanesulfonate using freshly prepared versus cryopreserved tissues
Environ. Mol. Mutagen., 53 (2012), pp. 101-113
Salim et al., 2004
E.I. Salim, M. Kaneko, H. Wanibuchi, K. Morimura, S. Fukushima
Lack of carcinogenicity of enzymatically modified isoquercitrin in F344/DuCrj rats
Food Chem. Toxicol., 42 (2004), pp. 1949-1969
Shimada et al., 2010
Y. Shimada, Y. Dewa, R. Ichimura, T. Suzuki, S. Mizukami, S.M. Hayashi, M. Shibutani, K. Mitsumori
Antioxidant enzymatically modified isoquercitrin suppresses the development of liver preneoplastic lesions in rats induced by beta-naphthoflavone
Toxicology, 268 (2010), pp. 213-218
Smith et al., 2005a
R.L. Smith, S.M. Cohen, J. Doull, V.J. Feron, J.I. Goodman, L.J. Marnett, I.C. Munro, P.S. Portoghese, W.J. Waddell, B.M. Wagner, T.B. Adams
Criteria for the safety evaluation of flavoring substances. The Expert Panel of the flavor and extract manufacturers association
Food Chem. Toxicol., 43 (2005), pp. 1141-1177
Smith et al., 2005b
R.L. Smith, S.M. Cohen, J. Doull, V.J. Feron, J.I. Goodman, L.J. Marnett, P.S. Portoghese, W.J. Waddell, B.M. Wagner, T.B. Adams
GRAS flavoring substances 22
Food Technol., 59 (2005)
Tamano et al., 2001
S. Tamano, Y. Hatahara, M. Sano, A. Hagiwara, M. Nakamura, T. Wahino, K. Imaida
13-Week oral toxicity and 4-week recovery study of enzymatically modified isoquercitrin in F344/DuCrj rats
Jpn. J. Food Chem., 8 (2001), pp. 161-167
Utesch et al., 2008
D. Utesch, K. Feige, J. Dasenbrock, T.H. Broschard, M. Harwood, B. Danielewska-Nikiel, T.C. Lines
Evaluation of the potential in vivo genotoxicity of quercetin
Mutat. Res., 654 (2008), pp. 38-44
Valentova et al., 2014
K. Valentova, J. Vrba, M. Bancirova, J. Ulrichova, V. Kren
Isoquercitrin: pharmacology, toxicology, and metabolism
Food Chem. Toxicol., 68 (2014), pp. 267-282
Witt et al., 2008
K.L. Witt, E. Livanos, G.E. Kissling, D.K. Torous, W. Caspary, R.R. Tice, L. Recio
Comparison of flow cytometry- and microscopy-based methods for measuring micronucleated reticulocyte frequencies in rodents treated with nongenotoxic and genotoxic chemicals
Mutat. Res., 649 (2008), pp. 101-113
Yoshida et al., 2017
T. Yoshida, H. Murayama, M. Kawashima, R. Nagahara, Y. Kangawa, S. Mizukami, M. Kimura, H. Abe, S.M. Hayashi, M. Shibutani
Apocynin and enzymatically modified isoquercitrin suppress the expression of a NADPH oxidase subunit p22phox in steatosis-related preneoplastic liver foci of rats
Exp. Toxicol. Pathol., 69 (2017), pp. 9-16