Myricitrin, a flavonol rhamnoside of myricetin extracted from the Chinese bayberry (Myrica rubra Siebold) plant, has been used in Japan since 1992 as a flavour modifier in snack foods, dairy products, and beverages. It is affirmed as generally recognised as safe (GRAS) by the US Flavour and Extract Manufacturer Association (FEMA) and is considered safe by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) at current estimated dietary exposures. In anticipation of expanded marketing, 97% pure myricitrin was fed to male and female Sprague–Dawley rats at dietary concentrations of 0.5%, 1.5% and 5.0% in a 90-day toxicity study. There was increased food consumption and decreased body weight gain in males exposed to 5% myricitrin. Blood values were within laboratory reference ranges except for mean increases in basophils in low- and high-dose males and serum phosphorus in high-dose males. In the absence of abnormal clinical or histopathological changes, these changes are not considered adverse. Based on the 90-day rat toxicity study, the no observed adverse effect level (NOAEL) is 2926 mg kg–1 day–1 in males and 3197 mg kg–1 day–1 in females. Gavage administration of myricitrin resulted in blood levels of myricitrin within 1 h after single oral doses of 250, 500 or 1000 mg kg–1 body weight, indicating direct absorption of the glycosylated form of this flavonoid. Blood levels of myricetin, a metabolite of myricitrin, were not present in rats dosed orally with 1.6 mg kg–1 myricetin, but were present only at 12 or 24 h in one of five, in three of five, and in four of five rats dosed with 250, 500 and 1000 mg myricitrin kg–1 body weight, respectively, possibly a result of hepatic conversion of myricitrin to myricetin and enterohepatic recirculation of the resulting myricetin. The current studies further support prior safety assessments of myricitrin as a food flavouring.
Keywords
flavour modifier, flavonol, myricetin, GRAS
Introduction
Myricitrin is the rhamnose glycoside of myricetin, a naturally occurring flavonol isolated from the fruit, bark or leaves of the Chinese bayberry (Myrica rubra Siebold) and other medicinal plants. As with other polyphenols, it has a long history of use for its anti-oxidant, free-radical scavenging, anxiolytic, antinociceptive and anti-inflammatory properties and has been available from the Japanese market since 1992. The pharmacological properties are largely based on the inhibition of subtypes of protein kinase C, p38MAPK signalling, and inhibition of nitric oxidase synthetase, cyclooxygenase 2, TNF alpha, and myeloperoxidase activities (Schwanke et al. 2013). In Japan myricitrin is used as a flavour modifier in snack food, dairy products and beverages. Myricetin, the aglycone of myricitrin, structurally differs from quercetin by the presence of a hydroxyl group at the 5ʹ position on the B ring. The structures of myricitrin, myricetin and quercetin are present in Figure 1.
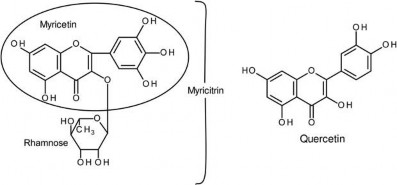
Figure 1. Structural formulas for myricitrin, myricetin and quercetin.
As a flavonoid,the bioavailability of dietary myricitrin is expected to occur following hydrolysis with release of the aglycone (myricetin) in the small intestine (Cook & Karmazyn 1996; Bravo 1998; Aherne & O’Brien 2002; Del Rio et al. 2010). Flavonoids and metabolites not absorbed in the small intestine are then acted on by colonic microflora with cleavage of conjugating moieties and ring fission of aglycones, absorption of resultant phenolic acids, phase II hepatic metabolism with enterohepatic circulation and urinary excretion (Blaut et al. 2003; Del Rio et al. 2010).
For myricitrin specifically, early studies in rats show that intestinal microorganisms breakdown myricitrin to myricetin, 3,4,5-trihydroxyphenylacetic acid and 3,5-dihydroxyphenylacetic acid (Smith & Griffiths 1970) with urinary excretion of ring-fission hydroxyphenylacetic products (Griffiths & Smith 1972). Once absorbed, the liver is the main site of myricetin metabolism while intestinal wall and kidney are secondary sites of metabolism (Ong & Khoo 1997). In a more recent study using intestinal loops in anaesthetised rats, myricitrin was absorbed by intestinal epithelial cells as a glycoside, not as the aglycone, with release into mesenteric blood as glucuronide or sulphate conjugates of myricitrin (Matsukawa et al. 2012). In simulated digestion experiments mimicking the human gastrointestinal system, myricitrin is stable at acidic pH 1.8 conditions and is degraded at alkaline pH 8.5, but without losing its inhibitory effect on induced low-density lipoprotein oxidation (Yokomizo & Moriwaki 2005). Myricitrin metabolites produced in vitro by human intestinal bacteria are indicative of dehydroxylation to quercetin-3-O-rhamnoside and deglycosylation to quercetin and myricetin (Du et al. 2014).
Myricitrin is affirmed as generally recognised as safe (GRAS) by the US Flavour and Extract Manufacturer Association (FEMA; Smith et al. 2009) and is considered to be of no safety concern based on current estimated dietary exposures by JECFA (2014). Highly purified commercially available myricitrin contains a small amount of myricetin, the aglycone of myricitrin. Myricetin and structurally related flavonoids such as quercetin have shown genotoxicity and DNA damage in several studies (Hardigree & Epler 1978; MacGregor & Jurd 1978; Hatcher & Bryan 1985; Sahu & Gray 1993; Hobbs et al. 2015). Previously conducted 3- and 12-month dietary studies of Chinese bayberry extract in rats were without toxicity (Yoshino et al. 2001). However, in those studies the test material was only 29.8% pure and contained 0.44% myricetin. Thus, the actual exposure to myricitrin may not have been sufficiently high enough to ensure safety. In anticipation of an expanded market as a flavouring agent, a 90-day rat toxicity study and a single-dose toxicokinetic (TK) study to define the safety of > 97% pure myricitrin are reported herein.
Materials and methods
Ninety-day repeated-dose toxicity study
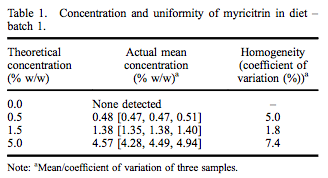
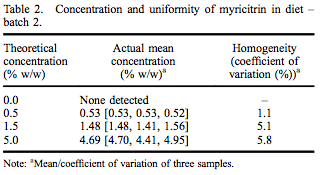
A repeated-dose 90-day oral toxicity study was conducted at Integrated Laboratory Services (ILS), Inc. (Research Triangle Park, NC, USA) in male and female Sprague– Dawley rats following good laboratory practices, Organisation for Economic Co-operation and Development (OECD) Guideline #408, and Eika No. 29 (Japan Ministry of Health and Welfare). Greater than 97% pure myricitrin, containing 0.16% myricetin, was obtained from San-Ei Gen, F.F.I., Inc. (Osaka, Japan) and added to Purina Certified 5002 meal diet (Ralston Purina Company, St. Louis, MO, USA) at dose concentrations of 0.5%, 1.5% and 5.0%. Dose formulations were within acceptable criteria for both concentration and uniformity (Tables 1 and 2). Stability of myricitrin in the diet stored between 0 and 30° C was at least 56 days. Control diet was Purina Certified 5002 meal without added myricitrin. Ten males and 10 females were assigned to each dietary group based on equivalent body weight following a 7-day acclimatisation period. Dose levels were selected following a 14-day range-finding study without abnormal clinical observations, changes in body weights or selected tissue weights.
Toxicokinetic (TK) study
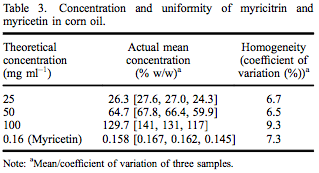
A TK study of myricitrin (> 97% pure as above) was conducted at ILS in accordance with the USFDA’s Good Laboratory Practice Regulations (21 CFR Part 58). This study was designed to satisfy the Testing Guideline No. 417: Toxicokinetics (OECD 2010), specifically timecourse studies; plasma/blood kinetics, as well as all applicable standard operating procedures of ILS. Twenty male Sprague–Dawley rats were allocated to one of four designated dose groups and administered a single oral dose of one of three dose levels of myricitrin (250, 500 and 1000 mg kg–1 body weight) or one dose level of myricetin (1.6 mg kg–1 body weight) in corn oil. The concentration and uniformity of myricitrin and myricetin in corn oil was within acceptable limits (Table 3). The top dose of myricitrin (1000 mg kg–1 body weight) was selected due to the low toxicity and the limit dose as stipulated in the test guideline (OECD 2010). Because myricetin, the aglycone of myricitrin, has some demonstrated genotoxicity, one group of rats was administered 1.6 mg kg–1 myricetin based on the amount of myricetin present in the highest dose of myricitrin. Blood was collected prior to and at 1, 3, 6, 12 and 24 h following dose administration. Plasma was analysed for myricitrin and myricetin concentrations by Applied Biosystems API-3000 LC/MS/MS (Applied Biosystems, Grand Island, NY, USA).
Animal care
Both studies were conducted within the same Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited specific pathogen-free facility. All procedures were in compliance with Animal Welfare Act Regulations (9CFR 1-4) and the Guide for the Care and Use of Laboratory Animals (ILAR 2011), and with approval of the ILS Institutional Animal Care and Use Committee.
Statistical analysis
Group mean and standard deviations (SDs) were calculated and reported. All data were analysed – final body weight, body weight gain, food consumption (g kg–1 day–1 ), absolute and relative (to body weight) tissue weights, neurotoxicological endpoints, urinalysis endpoints, and clinical pathology endpoints – using Statistical Analysis System version 9.2 (SAS Institute, Cary, NC, USA). First, studentised residual plots were used to detect possible outliers in the data. Homogeneity of variance was analysed using Levene’s test. If the data were heterogeneous, then appropriate transformations (log, square root, multiplicative inverse) were performed and the data reanalysed for homogeneity of variance. Data were then analysed using a one-way analysis of variance (ANOVA) and myricitrinexposed groups were compared with the appropriate control group using Dunnett’s test. Heterogeneous datasets were analysed using a Dunn’s test to compare exposed groups with the concurrent control group. Finally, dosedependent changes were evaluated using a linear regression model.
TK parameters were derived using non-compartmental methods employing a validated installation (validated on 20 September 2013) of Phoenix WinNonlin® version 6.3 (Pharsight Corporation, St. Louis, MO, USA). Area under the curve (AUC) values for myricitrin concentration of each animal were determined and the resulting values were compared among groups via appropriate t-tests.
Results
Ninety-day repeated-dose study
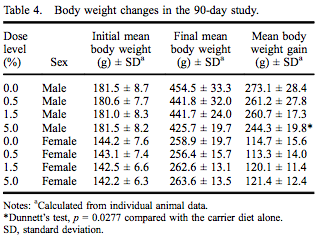
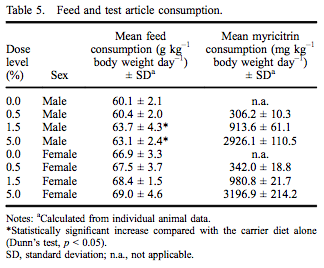
All rats survived to the scheduled termination at 90–93 days without any clinical evidence of moribundity. There were no myricitrin-related clinical abnormalities during the study. Group mean initial and final body weights as well as body weight gain are presented in Table 4. While there were no statistically significant changes in final body weight, there was a 10.5% reduced body weight gain in males in the 5.0% dose group. There were no changes in feed consumption in females exposed to myricitrin compared with concurrent controls. There was a significant increase in food consumed by male rats exposed to 1.5% and 5.0% myricitrin compared with concurrent controls with a significant dose-dependent trend (Table 5).
Prior to dosed-diet exposure, ocular examinations found six male and four female rats with mild corneal crystals. Examination of control and high-dose group animals within 1 week of study termination found mild corneal crystals in three male and two female rats in the control group and in five male and four female rats in the high-dose group. No other abnormalities were observed. Corneal crystals are a common finding in Sprague– Dawley rats and were not considered to have a causal relationship with myricitrin exposure. With respect to neurotoxicity screening, the functional operation battery evaluation and automated motor activity assessments of animals showed no significant changes in myricitrinexposed animals compared with concurrent controls (data not shown). There were no effects on oestrous cyclicity in treated versus control females during 5 consecutive days immediately preceding termination (data not shown). There were no significant changes in urinalysis parameters in male or female rats exposed to myricitrin compared with concurrent controls.
Minor gross observations were seen in one control, one 0.5% and three 5.0% females. These were subsequently identified histologically as minimal non-adverse lymphoid enlargements representing Peyer’s patches in the intestines.
The majority of tissues examined showed no changes in their absolute and relative weights (relative to body weight) when compared with concurrent controls. Tissues with no changes in either gender compared with those of the concurrent control group included: adrenals, brain, lungs, salivary glands, thymus, epididymides, prostate, seminal vesicles, testes, ovaries and uterus with cervix.
Significant decreases in absolute, but not relative, male liver (88% of control) and thyroid (81% of control) weights were observed in male rats exposed to 5.0% myricitrin compared with the carrier diet alone (Figure 2). Absolute pituitary weights were significantly decreased in male rats exposed to 0.5% or 5.0% (86% and 89% of control, respectively) myricitrin compared with animals given carrier diet alone; however, no differences in pituitary weights relative to body weight were observed (Figure 2). There was a corresponding significant decreasing dose-dependent trend in male pituitary weights. Absolute and relative spleen (90% and 89% of control, respectively), relative heart (92% of control), and relative kidney (93% of control) weights were significantly decreased in female rats exposed to 5.0% myricitrin compared with the concurrent control animals (Figure 3).
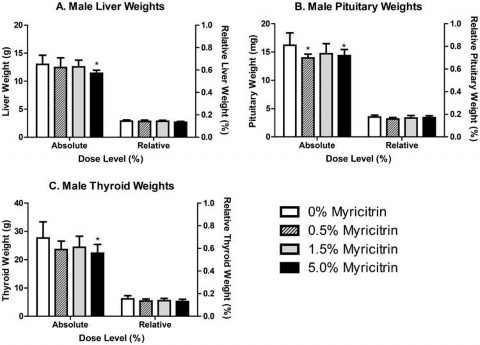
Figure 2. Absolute and relative weights of (A) liver, (B) pituitary and (C) thyroid from male Sprague–Dawley rats at the end of the 90- day study. Relative weights are relative to body weight. Bars represent the mean ± standard deviation (SD) of 9–10 rats/group. Statistically significant decrease compared with carrier diet alone. Dunnett’s test, p < 0.05.
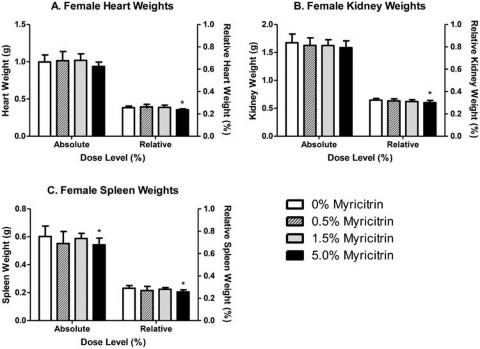
Figure 3. Absolute and relative weights of (A) heart, (B) kidney and (C) spleen from female Sprague–Dawley rats at the end of the 90- day study. Relative weights are relative to body weight. Bars represent the mean ± standard deviation (SD) of 9–10 rats/group. Statistically significant decrease compared with carrier diet alone. Dunnett’s test, p < 0.05.
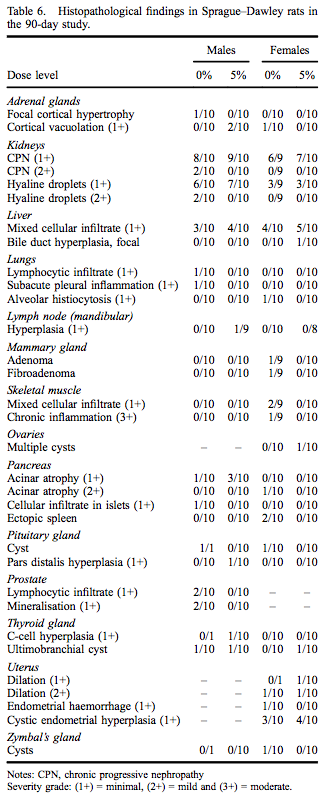
Administration of myricitrin in feed to male and female Sprague–Dawley rats for 90–93 days was not associated with any definitive microscopic findings attributable to myricitrin exposure based on examination of high-dose and control groups. Histopathological findings, all of which are commonly observed spontaneous changes in 90-day rat studies, are presented in Table 6.
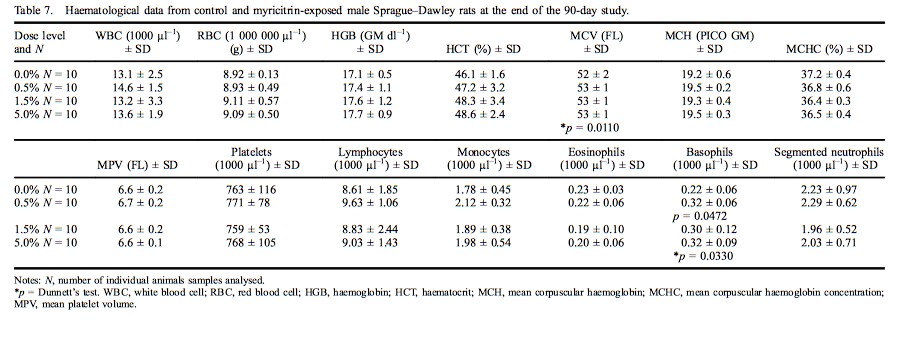
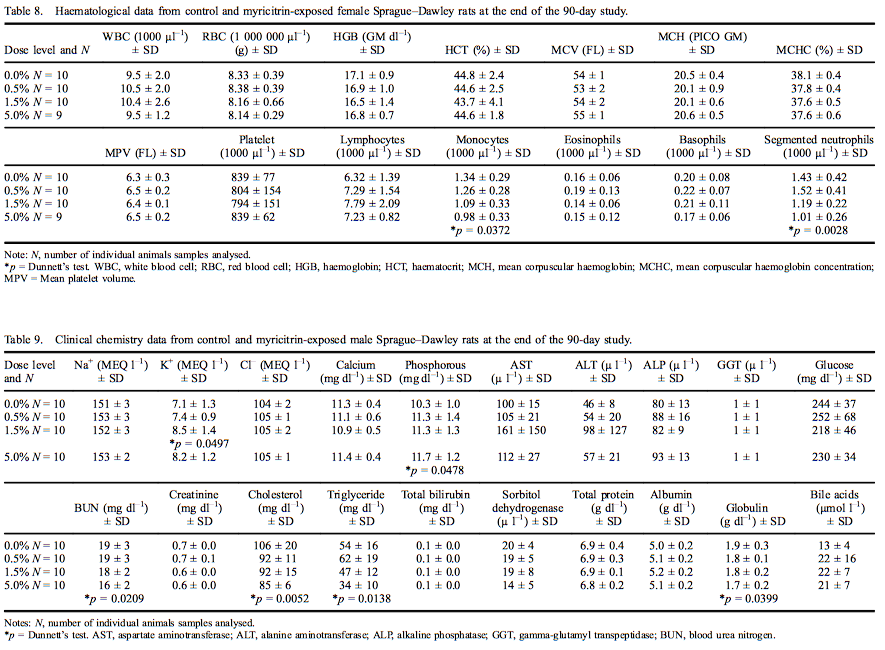
Mean haematological values for males are presented in Table 7. A statistically significant increase was measured in mean corpuscular volume (MCV) in male rats exposed to 5.0% myricitrin (103% of control) as compared with the concurrent controls. The MCV changes were all within the performing laboratory reference ranges. Statistically significant increases in basophils were present in male rats exposed to 0.5% or 5.0% myricitrin (143% and 146% of controls, respectively). Mean haematological values for females are presented in Table 8. Segmented neutrophils (70% of control) and monocytes (73% of control) were significantly decreased in female rats administered 5.0% myricitrin. Female rat lymphocyte levels exhibited a statistically significant increasing dose-dependent trend without significant changes in any dose group compared with the concurrent control. Segmented neutrophil and monocyte values fell within the reference ranges of the performing laboratory.
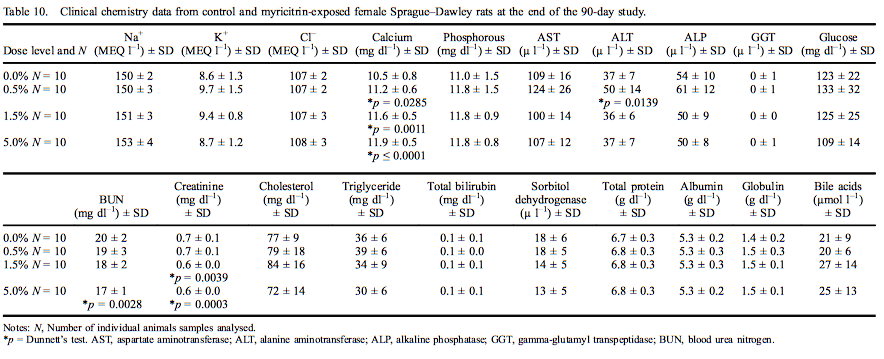
Clinical chemistry analyte mean values for males are presented in Table 9. The level of phosphorous (113% of control) was statistically significantly increased; and urea nitrogen (85% of control), cholesterol (80% of control), triglycerides (64% of control) and globulin (88% of control) were significantly decreased in male rats exposed to 5.0% myricitrin compared with concurrent controls. Potassium levels were significantly increased (119% of control) in male rats exposed to 1.5% myricitrin. Creatinine levels were significantly decreased in males exposed to 1.5% and 5.0% (90% and 87% of control, respectively) myricitrin compared with the concurrent controls. A statistically positive dose-dependent decrease in bile acids was noted; however, there were no significant pairwise comparisons between myricitrin-exposed animals and concurrent controls. All the above statistically significant clinical chemistry levels were within the reference ranges of the performing laboratory, except for the increased phosphorous levels that fell slightly outside the reference range.
Female rat clinical chemistry analytes are presented in Table 10. Calcium levels were significantly elevated at all dose levels (11.8% in females exposed to 5.0% myricitrin) with an increasing dose-dependent trend. ALT was significantly increased (135% of control) in female rats exposed to 0.5% myricitrin compared with the concurrent controls. Urea nitrogen was significantly decreased (83% of control) in female rats administered 5.0% myricitrin while creatinine was significantly decreased in female rats administered 1.5% and 5.0% myricitrin (88% and 85% of control, respectively). All the above significantly affected clinical chemistry levels were within the reference ranges of the performing laboratory.
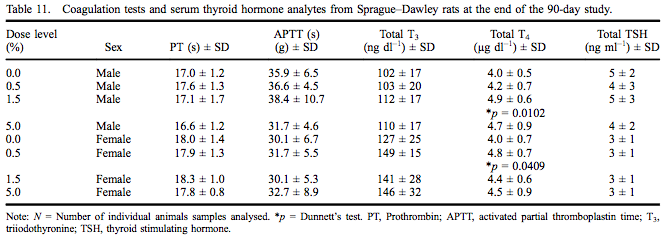
Coagulation test data and thyroid hormone levels are presented in Table 11 for both sexes. Prothrombin and APTTs did not differ among groups and were within the testing laboratory reference range for this age of rats. Serum T4 levels were statistically significantly increased (124% of control) at 1.5% myricitrin administration in male rats with a significant dose-dependent increase. The serum T4 level was significantly increased (121% of control) in female rats exposed to 0.5% myricitrin. All serum T4 values for male and female rats were within the performing laboratory reference range.
Toxicokinetic (TK) study
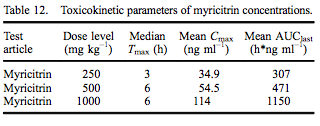
Using jugular implants, blood was collected from five rats in each dose group at 0 (pre-dose), 1, 3, 6, 12 and 24 h following gavage administration of myricitrin or myricetin in corn oil. The 1.6 mg kg–1 body weight of myricetin was based on the amount of myricetin present in the highest dose of myricitrin (1000 mg kg–1 ). All animals survived to termination without signs of moribundity, abnormal clinical signs or abnormal body weight changes. Myricitrin TK parameters are presented in Table 12. Median Tmax was at 3 h for rats dosed with 250 mg kg–1 myricitrin and 6 h for rats dosed with 500 or 1000 mg kg–1 myricitrin. Mean Cmax and AUClast values increased approximately proportionally to dose level.
Administration of myricitrin resulted in quantifiable concentrations of myricetin in the blood. Myricetin plasma concentrations were observed in rats administered 250 mg kg–1 (1/5 rats), 500 mg kg–1 (3/5 rats), and 1000 mg kg–1 (4/5 rats) myricitrin only at 12 and/or 24 h post-dose. Myricetin concentration data generated from animals administered myricitrin were not analysed for TK parameters as there were not enough quantifiable concentrations over the 24-h collection period to provide meaningful results. Both myricitrin and myricetin were below the LOQ in rats administered 1.6 mg of myricetin kg–1 body weight, a dose equivalent to the concentration of myricetin present in the top myricitrin dose of 1000 mg kg–1 .
Discussion
Ninety-day repeated-dose study
The 90-day repeated-dose toxicity study was undertaken because of significant increases in the purity of myricitrin as compared with the myricitrin used in a previous 90-day and 52-week dietary study (Yoshino et al. 2001). The myricitrin used in the Yoshino study was 28.8% pure and contained 0.44% myricetin, the aglycone of myricitrin. The myricitrin used in the present 90-day study was 97% pure and contained 0.16% myricetin.
The statistically significant reduced body weight gain and non-statistical decreased final body weight in males consuming the 5% myricitrin diet considered in light of a statistically significant increased food consumption in the mid- and high-dose males suggests a decreased efficiency of feed utilisation. All other clinical observations remained normal throughout the study and ophthalmological and histopathological changes were within normal background levels for the age and strain of rats in the study. In the light of no treatment-related histopathological changes in any organ, the reduced body weight gain in the high-dose males is judged as not adverse.
There were several decreases in tissue organ weights primarily including liver, thyroid and pituitary in 5.0% group males and spleen, heart and kidneys in 5.0% group females. In general these reductions were minor and none was accompanied by histopathological changes or altered relevant clinical chemistry analytes. The decreased organ weights may be related to reduced caloric intake or nutrient displacement by the high level of dietary myricitrin. The majority of measured haematological and serum study parameters did not differ significantly between myricitrin-exposed rats and concurrent controls; and when statistically significant, were within reference values of the performing laboratory. The exceptions were an increase in basophils in the WBC differential cell count for 0.5% and 5.0% group males and serum phosphorus in the 5.0% male group. There is no apparent explanation for the elevated basophil count that was dose independent, and the serum phosphorus level was marginally significant and only slightly outside the control reference value of the performing laboratory. Furthermore, these two changes are not related, are relatively minor and are not consistent across both genders. Consequently, the differential basophil cell count and the slightly increased serum phosphorus level are not regarded as adverse.
Toxicokinetic (TK) study
Following gavage administration of 250 mg kg–1 and greater myricitrin in corn oil, blood levels of myricitrin without deglycosylation were detected within 1 h in all rats. This was an unexpected finding. In published accounts of myricitrin metabolism, as well as metabolism of structurally related flavonoids, blood levels are typically glucuronide and/or sulphate conjugates of the flavonoid aglycone. There are a couple of notable exceptions, however. Using ligated intestinal loops in anaesthetised rats, absorption of myricitrin as the glycoside, not the aglycone, was demonstrated via transcellular transport (Matsukawa et al. 2012). In contrast to our findings, Matsukawa and co-workers identified myricitrin glucuronide or sulphate in mesenteric blood and not unconjugated myricitrin glycoside. They propose that myricitrin glycoside was taken up by the glucose transporter, GLUT2, with transfer to the epithelial apical brush border and incorporation into the epithelium by endocytosis. There is some experimental evidence for direct glycoside absorption with a related flavonoid glycoside, quercetin, employing chambers with rat intestinal mucosa (Wolffram et al. 2002). In addition, using ligated jejunal and ileal intestinal loops, the highly soluble quercetin glycoside, alpha-G-rutin, resulted in appearance of intact glycoside as well as quercetin and conjugated quercetin in mesenteric blood (Matsumoto et al. 2007). We believe that the blood levels of myricitrin in our study reflect direct absorption in the small intestine without deglycosylation, similar to what has been shown with quercetin glycoside (Wolffram et al. 2002; Matsumoto et al. 2007), and that this response is, in part, a consequence of using > 97% pure myricitrin in this study. Concomitant circulating conjugated forms of myricitrin may have also been present following metabolism in the liver. However, these were not measured in the present study. Any of the dosed myricitrin that may have reached the large intestine would most probably have undergone glycosidic cleavage by anaerobic bacteria yielding the ring-fission products 3-hydroxyphenylacetic acid and 3,5-dihydroxyphenylacetic acid (Smith & Griffiths 1970; Griffiths & Smith 1972).
Myricetin is the active form of myricitrin and has some genotoxic activity (Hardigree & Epler 1978; MacGregor & Jurd 1978; Hatcher & Bryan 1985; Sahu & Gray 1993; Hobbs et al. 2015). Quantifiable blood levels of myricetin were observed in 1/5, 3/5 and 4/5 rats dosed with 250, 500 and 1000 mg kg–1 of myricitrin, respectively. A group of rats was dosed with 1.6 mg kg–1 myricetin based on the amount of myricetin present in the highest dose (1000 mg kg–1 ) of myricitrin. Since blood levels of myricitrin and myricetin were below the LOQ at all times tested, we believe that this small amount of administered myricetin was either broken down in the gastrointestinal tract or rapidly conjugated and metabolised. The fact that myricetin was detected in blood of some rats at 12 and/or 24 h post-dosing with myricitrin is likely a consequence of hepatic conversion of myricitrin to myricetin and perhaps enterohepatic recirculation of the resulting myricetin.
Conclusions
Administration of > 97% pure myricitrin resulted in quanti- fiable blood concentrations of myricitrin within 1 h following oral administration indicating direct absorption of the glycosylated form of this flavonoid. Blood levels of myricetin were below the LOQ in rats dosed orally with 1.6 mg kg–1 myricetin, but were present in 20–80% of rats, increasing with dose level, dosed with myricitrin. The FEMA GRAS status and positive JECFA safety opinion of myricitrin attest to its safety at current use levels. The present 90-day rat toxicity study using > 97% pure myricitrin further supports the safety of myricitrin as a food flavouring. Based on the 90-day rat toxicity study, the no observed adverse effect level (NOAEL) is 2926 mg kg–1 day–1 in males and 3197 mg kg–1 day–1 in females.
Acknowledgements
The authors thank the investigative toxicology staff and the necropsy and histology teams of Integrated Laboratory Systems, Inc., for technical support, and Nuventra Pharma Sciences for toxicokinetic support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
ILS, Inc., was responsible for the study design, collection, analysis and interpretation of data and for the preparation of the final study report. The manuscript was prepared by R. Maronpot. The decision to submit the paper for publication was made by San-Ei Gen, F.F.I., Inc.
References
Aherne SA, O’Brien NM. 2002. Dietary flavonols: chemistry, food content, and metabolism. Nutrition. 18:75–81.
Blaut M, Schoefer L, Braune A. 2003. Transformation of flavonoids by intestinal microorganisms. Int J Vitam Nutr Res. 73:79–87.
Bravo L. 1998. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 56:317–333.
Cook MA, Karmazyn M. 1996. Cardioprotective actions of adenosine and adenosine analogs. EXS. 76:325–344.
Del Rio D, Borges G, Crozier A. 2010. Berry flavonoids and phenolics: bioavailability and evidence of protective effects. Br J Nutr. 104:S67–S90.
Du L-Y, Zhao M, Xu J, Qian D-W, Jiang S, Shang E-X, Guo J-M, Liu P, Su S-L, Duan J-A, et al. 2014. Identification of the metabolites of myricitrin produced by human intestinal bacteria in vitro using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Expert Opin Drug Metab Toxicol. 10:921–931.
Griffiths LA, Smith GE. 1972. Metabolism of myricetin and related compounds in the rat. Metabolite formation in vivo and by the intestinal microflora in vitro. Biochem J. 130:141–151.
Hardigree AA, Epler JL. 1978. Comparative mutagenesis of plant flavonoids in microbial systems. Mutat Res. 58:231–239.
Hatcher JF, Bryan GT. 1985. Factors affecting the mutagenic activity of quercetin for Salmonella typhimurium TA98: metal ions, antioxidants and pH. Mutat Res. 148:13–23.
Hobbs CA, Swartz C, Maronpot RR, Davis J, Recio L, Hayashi S. 2015. Genotoxicity evaluation of the flavonoid myricitrin and it aglycone myricetin. Food Chem Toxicol. 83:283–292.
ILAR. 2011. Guide for the care and use of laboratory animals. 8th ed. Washington (DC): The National Academies Press; 220 p.
JECFA. 2014. Food additives and flavorings. 79th Meeting, Geneva. Available from: http://www.fao.org/food/foodsafety-quality/scientific-advice/calls-data-experts/en/
MacGregor JT, Jurd L. 1978. Mutagenicity of plant flavonoids: structural requirements for mutagenic activity in Salmonella typhimurium. Mutat Res. 54:297–309.
Matsukawa N, Matsumoto M, Hara H. 2012. 35. Nondigestible saccharide enhances transcellular transport of myricetin glycosides in the small intestine of rats. A newly defined mechanism of flavonoid absorption. In: Cho S, Almeida N, editors. Dietary fiber and health. New York: CRC Press; p. 487–496.
Matsumoto M, Matsukawa N, Chiji H, Hara H. 2007. Difructose Anhydride III promotes absorption of the soluble flavonoid αG-rutin in rats. J Agric Food Chem. 55:4202–4208.
OECD. 2010. OECD guideline for the testing of chemicals. Toxicokinetics. [Internet]. OECD 417. [cited 2010 Jul 22]. Available from: www.oecd-library.org
Ong KC, Khoo H-E. 1997. Biological effects of myricetin. Gen Pharmacol. 29:121–126.
Sahu SC, Gray GC. 1993. Interactions of flavonoids, trace metals, and oxygen: nuclear DNA damage and lipid peroxidation induced by myricetin. Cancer Lett. 70:73–79.
Schwanke RC, Marcon R, Meotti FC, Bento AF, Dutra RC, Pizzollatti MG, Calixto JB. 2013. Oral administration of the flavonoid myricitrin prevents dextran sulfate sodiuminduced experimental colitis in mice through modulation of PI3K/Akt signaling pathway. Mol Nutr Food Res. 57:1938–1949.
Smith GE, Griffiths LA. 1970. Metabolism of myricitrin and 3,4,5-trihydroxyphenylacetic acid. Biochem J. 118:53P–54P.
Smith R, Waddell W, Cohen S, Vj F, Marnett L, Portoghese P, Rietjens I, Adams T, Lucal Gavin C, McGowen M, et al. 2009. GRAS Flavoring Substances 24. Food Technology. 06:46–105.
Wolffram S, Block M, Ader P. 2002. Quercetin-3-glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J Nutr. 132:630–635.
Yokomizo A, Moriwaki M. 2005. Transepithelial permeability of myricitrin and its degradation by simulated digestion in human intestinal Caco-2 cell monolayer. Biosci Biotechnol Biochem. 69:1774–1776.
Yoshino H, Kawabe M, Tamano S, Hagiwara A, Washino T, Nakamura M, Imaida K. 2001. 1-year oral toxicity study of Chinese bayberry extract in F344/DuCrj rats. Jpn J Food Chem. 8:94–99.