The plant Angelica keiskei contains two main physiologically active flavonoid chalcones, 4-hydroxyderricin and xanthoangelol. Known as ashitaba in Japan, powder from the sap is widely consumed for its medicinal properties in Asia as a dietary supplement. Limited previously reported mammalian studies were without evidence of toxicity. GLP studies reported here, including a bacterial reverse mutation assay, a chromosome aberration assay, and an in vivo micronucleus assay are negative for genotoxicity. A GLPcompliant 90-day repeated oral gavage study of ashitaba yellow sap powder containing 8.45% chalcones in Sprague Dawley rats resulted in expected known physiological effects on coagulation parameters and plasma lipids at 300 and 1000 mg/kg/day. Ashitaba-related pathology included a dose-related male ratspecific alpha 2-urinary globulin nephropathy at 100, 300, and 1000 mg/kg/day and jejunal lymphangiectasia in both sexes at 1000 mg/kg/day. All other study parameters and histopathological changes were incidental or not of toxicological concern. Based on these studies ashitaba chalcone powder is not genotoxic with a NOAEL of 300 mg/kg in male and female rats.
Keywords
Chalcones, Flavonoids, Lymphangiectasia, Alpha 2-urinary globulin nephropathy, 4-Hydroxyderricin, Xanthoangelol
1. Introduction
The plant Angelica keiskei, native to the Japanese Izu Islands and the Izu, Bouso, and Miura peninsulas, is known in Japan as ashitaba, and is widely cultivated in Korea where it is known as sinsuncho or tomorrow leaf. The powder derived from the sap contains several flavonoid chalcones. The most abundant and physiologically active ashitaba chalcones are 4-hydroxyderricin and xanthoangelol. These chalcones have myriad health benefits from inducing apoptosis in cancer cells, to anti-oxidant, anti-inflammatory, anti-angiogenic, and anti-diabetic properties (Dimmock et al., 1999; Dorn et al., 2010). They have also been demonstrated to inhibit platelet aggregation (Lo et al., 2009), have vasorelaxant properties (Ko et al., 2004; Lin et al., 2001; Lo et al., 2009), and suppress differentiation of preadipocytes to adipocytes (Zhang et al., 2013). Ashitaba green tea is widely consumed in China, Japan, and India as a health-promoting drink; is a perennial herb in Korea as a vegetable juice ingredient; and ashitaba leaves have been consumed as food and medicine for many years on the Izu Islands in Japan (Chang et al., 2014; Enoki et al., 2007; Raj et al., 2013).
The safety of ashitaba chalcone in a 28-day dietary administration of 17, 170, and 1700 mg/kg body weight of ashitaba powder to male Wistar rats was without evidence of toxicity (Nagata et al., 2007). Absorption and metabolism of 4-hydroxyderricin and xanthoangelol following gavage administration of 50, 100, 200,and 500 mg/kg body weight of ashitaba extract in male ICR mice (Nakamura et al., 2012) was without adverse effect. A feeding study of xanthohumol, a related prenylated chalcone present in hops, was conducted in female BALB/c mice for three weeks, achieving a daily dose of approximately 1000 mg/kg body weight without toxicity (Dorn et al., 2010). To further document the safety profile of ashitaba, data from a battery of toxicity tests are presented.
2. Materials and methods
2.1. Studies reviewed
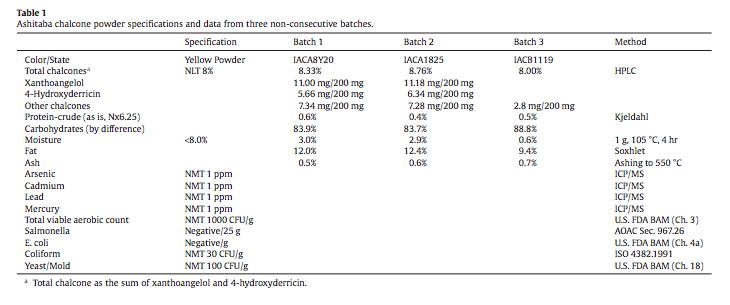
A battery of genotoxicity assays, an acute oral toxicity study in rats, and a 13- week rat subchronic oral toxicity study were contracted by Japan Bio Science Laboratory (JBSL), Osaka City, Japan. The test substance for all studies was provided by JBSL and prepared under Good Manufacturing Practices from plants organically cultivated in Indonesia. Ashitaba powder is obtained from the sap of cut stems. The sap is pasteurized, mixed with cyclodextrin, sterilized, freeze-dried, shattered and passed through 100 mesh to obtain 8% ashitaba powder. Analytical data from three non-consecutive batches are presented in Table 1. Data from the following studies are presented in this report by the author at the request of JBSL:
1. Bacterial reverse mutation test conducted at TNO Nutrition and Food Research Laboratory, The Netherlands (TNO Study Number 4405/16; 30 July 2002)
2. Chromosome aberration test conducted at TNO Nutrition and Food Research Laboratory, The Netherlands (TNO Study Number 5002/02; 3 June 2003)
3. In vivo mouse micronucleus test conducted at Nucro-Technics, Ontario, Canada (Study Number 282452; 13 June 2014)
4. Acute oral toxicity in rats conducted at TNO Nutrition and Food Research Laboratory, The Netherlands (TNO Study Number 4410/06; 31 May 2006)
5. 13-Week rat oral toxicity study conducted at Bozo Research Center, Japan (Study No. B-5530; 31 May 2006) with a subsequent independent pathology peer review (Experimental Pathology Laboratories, Inc., Research Triangle Park, NC; EPL Project No. 946-001; 27 March 2012).
2.2. Animal husbandry and care
The animal care and use committee at each performing laboratory approved study protocols. Animals were housed and maintained according to the AAALAC International Guide for the Care and Use of Laboratory Animals and CCAC Guidelines for Care and Use of Experimental Animals.
2.3. Study details
2.3.1. Bacterial reverse mutation assay
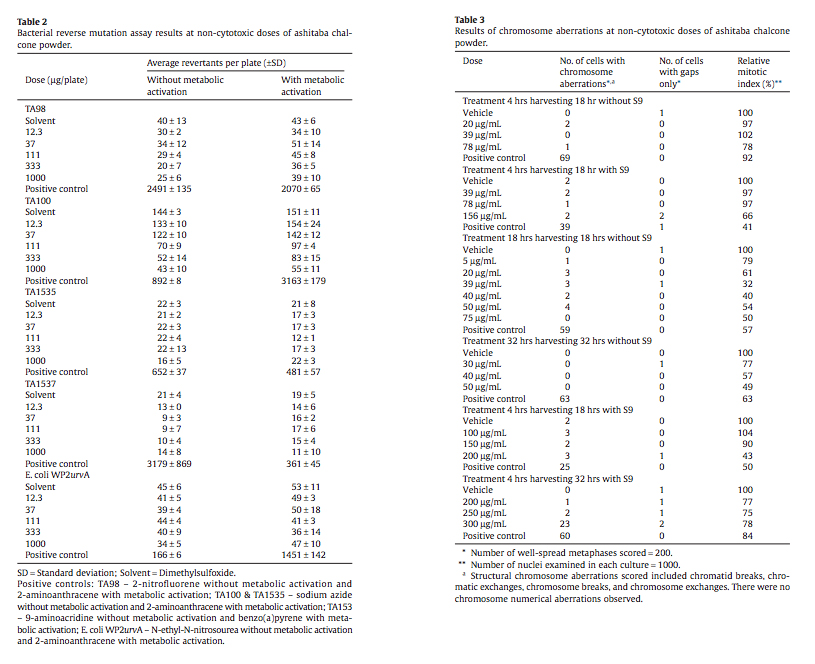
The bacterial reverse mutation assay was carried out in compliance with Good Laboratory Practices and OECD Guideline 471 utilizing Salmonella typhimurium strains TA 1535, TA 1537, TA 98, and TA 100 with tryptophan-requiring Escherichia coli strain WP2 uvrA. All assays were conducted with and without metabolic activation (S9 mix from liver homogenate from rats treated with Aroclor 1254). Ashitaba chalcone powder was cytotoxic in Salmonella strain TA 100 and negative for other strains tested both with and without metabolic activation. The positive controls (see Table 2) gave the expected response in revertant colonies with and without S9-mix. The highest concentration of test agent that could be dissolved in DMSO was 125 mg/mL. Given the 8% purity of chalcone, the highest concentration of chalcone was 10 mg/mL resulting in a highest concentration of 1000 μg/plate. The assay used 5 different concentrations of test substance from 12.3 to 1000 μg/plate. Assay results were considered positive if the mean number of revertant colonies had a concentrationdependent increase or if there was a reproducible two-fold increase over negative control.
2.3.2. Chromosome aberration
Two chromosome aberration tests were conducted in compliance with Good Laboratory Practice and OECD Guideline 473. Tissue culture media was purchased from Life Technologies (Gibco, The Netherlands) and Chinese hamster ovary cells (CHO K-1 line) were obtained from Prof. Dr. A.T. Natarajan, University of Leiden, The Netherlands. Other reagents included S9 mix (as above), colcemid (Fluka AG, Switzerland), DMSO (Sigma Chemical Company, USA), and methanol, acetic acid, and Giemsa stain (Merck-Darmstadt, FRG). The purity of the ashitaba powder was set at 100% (actual concentration of chalcone was 8%) and final concentrations of test substance in culture media ranged from 10 to 2500 μg/mL. Positive controls consisted of mitomycin C in the absence of S9 and cyclophosphamide in the presence of S9 mix. All cells at the highest concentrations (2500, 1250, 625 and 313 μg/mL) died before the end of a 4-hour treatment and these concentrations were considered too toxic for further study. For non-cytotoxic dose concentrations, a two-sided Fisher’s exact probability test was used to compare treated versus control. Study details are as follows:
Test 1. In the absence of S9 metabolic activation, treatment time was 4 hours (pulse treatment) and 18 hours (continuous treatment) while in the presence of S9 metabolic activation treatment time was 4 hours (pulse treatment). Harvesting of cells was 18 hours after onset of treatment.
Test 2. In the absence of S9 continuous treatment was for 18 hours with harvesting at 18 hours and at 32 hours. In the presence of S9 there was a 4-hour pulse exposure followed by harvesting at 18 and 32 hours. A repeat assay was done when the expected cyclophosphamide positive control did not yield expected results at a 32-hour sampling period.
2.3.3. In vivo MN assay
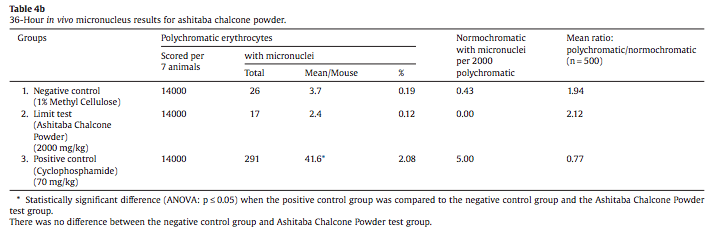
An in vivo micronucleus assay using ashitaba chalcone powder following OECD guideline 474 and in compliance with Good Laboratory Practices was conducted in Swiss mice (CD-1, Charles River, Canada). The range-finding study used groups of 3 male and female mice at doses of 500, 1000, and 2000 mg ashitaba chalcone power/ kg body weight. The ashitaba yellow powder consisted of 30% sap and 70% branched cyclodextrin and contained analytically confirmed 7.2% chalcone. Based on no sex difference in response in the range-finding study, the main study was done in groups of 14 male mice at the limit dose of ashitaba (2000 mg/kg), with a methylcellulose vehicle control, and 70 mg cyclophosphamide/kg body weight as a positive control. Seven mice in each group were terminated 24 and 36 hours post-dosing, harvested bone marrow was stained with Giemsa, and 2000 polychromatic erythrocytes were blindly scored by high magnification microscopy for the presence of micronuclei. The ratio of normochromatic to polychromatic erythrocytes was determined based on 500 cells and the number of micronuclei in normochromatic erythrocytes was also determined.
Any intergroup difference in mean number of polychromatic erythrocytes with micronuclei was analyzed using ANOVA on ranks (Kruskal–Wallis One Way Analysis of Variance on Ranks) at p < 0.05. To isolate the group(s) that differed from others, All Pair-wise Multiple Comparison Procedures (Dunn’s Method and Duncan’s Method) were used.
2.3.4. Acute toxicity
An acute gavage toxicity study of ashitaba chalcone powder (8% as chalcone) following OECD Guideline 423 and in compliance with Good Laboratory Practice was conducted in three male and three female Wistar outbred rats (Charles River, Germany) at a dose level of 2000 mg/kg following an overnight fast. Clinical signs and body weight were monitored during a 14-day observation period and rats were examined for macroscopic changes at study termination.
2.3.5. 90-day repeated dose oral toxicity study
A 90-day repeated dose oral gavage study of ashitaba chalcone was conducted in Sprague Dawley rats (Atsugi Breeding Center, Charles River, Japan) at 0, 100, 300 and 1000 mg/kg/day using 12 rats per dose per sex. Dose levels were selected based on result from a preliminary 14-day oral toxicity study. The ashitaba yellow sap powder (30.8% solids and 69.2% branched cyclodextrin) contained 8.45% chalcone and was suspended in olive oil for daily (7 days per week) gavage delivery using a dose volume of 5 mL/kg body weight. The study was done in compliance with Good Laboratory Practice and followed repeated dose toxicity study guidelines (No. 655, Ministry of Health and Welfare, Japan, April 5, 1999). Study parameters included daily clinical observations, frequent body weight measurements, food and water consumption, ophthalmological examinations, urinalysis, hematology, and clinical chemistry analyte measurements. At necropsy abnormal macroscopic findings were recorded and organ weights were obtained for brain, pituitary, thyroids (including parathyroids), adrenals, thymus, spleen, heart, lung, submandibular and sublingual salivary glands, liver, kidneys, testes, prostate, seminal vesicles, ovaries, and uterus. Histopathology slides for gross abnormalities and normal appearing tissues were stained with hematoxylin and eosin. Histopathology slides were peer reviewed by an experienced independent toxicologic pathologist (Seely, 2012).
Microscopic examination was done on gross lesions and all tissues from the high doses and controls. Potential test-article-related tissues examined microscopically from the middle- and low-dose groups of both sexes included adrenal, stomach, jejunum, and liver plus heart, cecum, and kidneys in males. Tissues examined microscopically from all high-dose and control rats included cerebrum, cerebellum, spinal cord (thoracic region), sciatic nerve, eyeballs, optic nerves, Harderian glands, pituitary, thyroids, parathyroids, adrenals, thymus, spleen, mandibular lymph node, mesenteric lymph node, heart, thoracic aorta, trachea, lung (with bronchus), tongue, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, submandibular and sublingual glands, liver, pancreas, kidneys, urinary bladder, testes, epididymides, prostate, seminal vesicle, ovary, uterus, vagina, oviduct, mammary gland, sternum (with bone marrow), femur (with bone marrow), femoral skeletal muscle, skin (inguinal region), nasal cavity, Zymbal’s glands and gross pathological abnormalities.
For statistical analyses, mean and standard deviation of body weight, food consumption, water consumption, quantitative measurements in urinalysis, hematology and clinical chemistry analytes and organ weights were calculated and analyzed for homogeneity of variance by Bartlett’s test (level of significance = 1%, two-tailed). For homogeneous data, group mean differences were analyzed by Dunnett’s test (level of significance: 5% and 1%, two-tailed). For heterogeneous data, the mean rank differences between groups were analyzed by a Dunnett-type mean rank test (levels of significance: 5% and 1%, two-tailed).
3. Results
3.1. Bacterial reverse mutation assay
Ashitaba chalcone powder was toxic to Salmonella TA100 both with and without metabolic activation as indicated by a decrease of revertant colonies versus vehicle control. All other bacterial strains were negative under conditions of the assay with vehicle and positive control values within acceptable ranges. Data are presented in Table 2.
3.2. Chromosome aberration
Test 1. Non-cytotoxic doses at and below 156 μg/mL were tested for chromosome aberrations. The 156 μg/mL dose reduced the mitotic index to 66% while lower doses were without effect on mitoses compared to the vehicle control. Pulse treatment with or without metabolic activation and 18 hours of continuous treatment without metabolic activation did not cause a statistically significant increase in aberrant cells at any of the concentrations tested (Table 3).
Test 2. Continuous treatment of cells for 18 and 32 hours without metabolic activation did not induce a statistically significant increase in aberrant cells at non-cytotoxic concentrations of ashitaba chalcone powder (Table 3). Short term treatment (4-hour pulsedose) with metabolic activation (S9 mix) did not induce statistically significant increases in aberrant cells at harvest times of 18 or 32 hours with one exception. A significant increase in aberrant cells was present at 32 hours for the 300 μg/mL dose. However, this dose exhibited transient cytotoxicity at 4 hours and was considered non-relevant by the laboratory conducting the assay.
3.3. In vivo MN
There was no effect on body weight or frequency of micronuclei in either polychromatic or normochromatic erythrocytes at 24 or 36 hours post-dosing at 2000 mg/kg (Tables 4a and 4b). While the ratio of polychromatic to normochromatic erythrocytes was normal for the ashitaba group, the decreased ratio at 36 hours versus 24 hours for the positive control is indicative of bone marrow suppression.
3.4. Acute toxicity
During a 14-day observation period following oral gavage of 2000 mg/kg ashitaba chalcone powder in aqueous solution, no abnormal clinical signs were observed. By the end of the 14-day observation period, all rats gained weight and were without treatment-related macroscopic alterations at study termination and necropsy. Based on these findings, ashitaba chalcone powder was considered not harmful if swallowed.
3.5. 90-day repeated dose toxicity study
3.5.1. Clinical observations
The study was well conducted with two early deaths consisting of one male control dead at week 12 with disseminated erythroblastic leukemia and one 300 mg/kg male dead at week 10 due to an intubation error. All remaining animals were without abnormal clinical signs throughout the study. Food consumption and body weight gain were comparable among all groups without any statistically significant differences. No ophthalmological abnormalities were present in any of the rats and urinalysis, including microscopic examination of sediment, revealed no statistically significant treatment-related effects.
3.5.2. Macroscopic findings and organ weights
Aside from the splenic enlargement and organ discoloration in a male control decedent and thoracic ascites in a 300 mg/kg group male decedent, the only macroscopic changes noted at terminal sacrifice were minor: red foci in lung, stomach, and liver involving single rats. The only organ weight change of possible relevance was increased relative kidney weight (combined kidneys) in high-dose (1000 mg/kg/day) males. This weight change was minor but may be a reflection of male rat-specific alpha 2-urinary globulin nephropathy seen in this group of rats.
3.5.3. Hematology
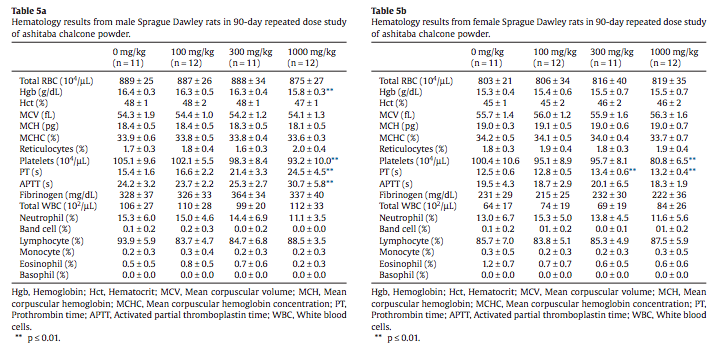
Most hemogram measurements were without treatmentassociated statistical effects and were within normal ranges for rats in typical 13-week studies. A statistically significant reduced hemoglobin value was flagged in high-dose males (Table 5a). Decreased platelet counts were present in high-dose male and female rats along with increased prothrombin time (PT) in male and female rats in the 300 mg/kg and 1000 mg/kg groups and increased activated partial thromboplastin time (APTT) in high-dose (1000 mg/kg/day) males (Tables 5a and 5b).
3.5.4. Clinical chemistry
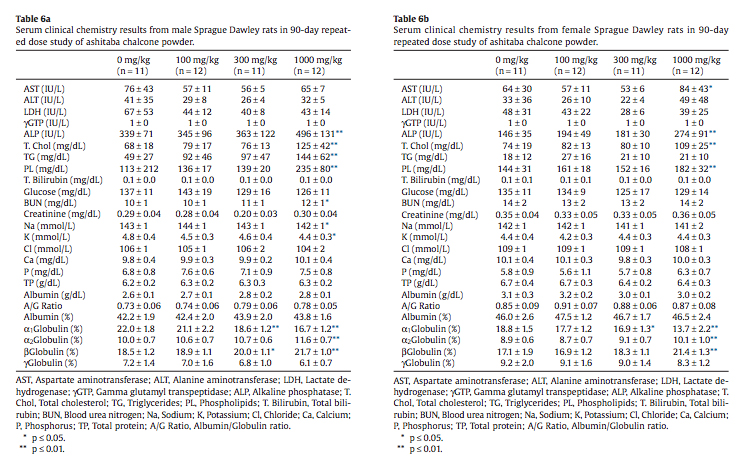
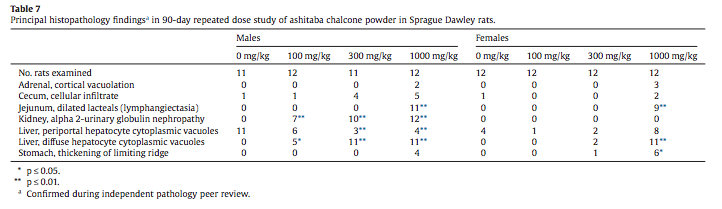
A broad spectrum of clinical chemistry analytes were measured with statistically significant increases in serum alkaline phosphatase, total cholesterol, and phospholipid in high-dose (1000 mg/kg/day) male and female rats and elevated serum triglyceride in high-dose males (Tables 6a and 6b). Very minor changes in blood urea nitrogen, serum sodium, and serum potassium in highdose males were statistically significant. Elevated serum total cholesterol, triglyceride and phospholipid were present as an expected finding.
3.5.5. Histopathology
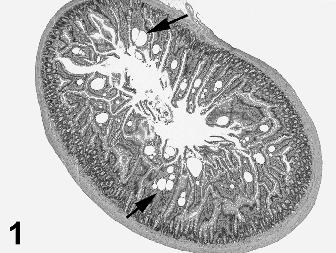
The histopathology findings from the 90-day study were peer reviewed by an independent pathologist (Seely, 2012) and subsequently also peer reviewed by R. Maronpot. Principal lesion incidences for potential treatment-related findings are presented in Table 7. Potential treatment-related effects that were con- firmed during peer review included minimal adrenal zona fasciculata cytoplasmic vacuolation in 2 males and 3 females in the 1000 mg/ kg groups, jejunal lacteal dilation (Fig. 1) in the 1000 mg/kg groups for both sexes and intracytoplasmic eosinophilic droplets and associated changes in renal proximal tubules consistent with a dose–response for alpha 2-urinary globulin nephropathy in treated male rats. Minimal thickening of the forestomach limiting ridge and minimal cellular infiltrates in the cecal lamina propria were diagnosed by the study pathologist (Table 7) but were considered within normal variation and not a test-article effect during the independent histopathology peer review (Seely, 2012).
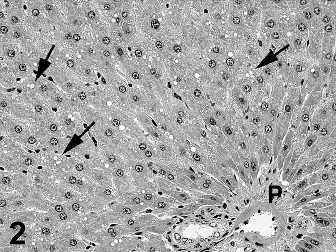
Two types of cytoplasmic vacuolation were reported in the liver with higher incidences in males than in females. Fine periportal hepatocyte cytoplasmic vacuoles (Fig. 2) were present in control and treated rats with a reduced incidence in Ashitaba-chalconeexposed males. Larger vacuoles consistent with intracytoplasmic fat in hepatocytes (Figs. 3 & 4) were present in treated males and females. The lobular localization of cytoplasmic vacuoles in treated rats was described as diffuse by the study pathologist and centrilobular by the peer review pathologist (Seely, 2012), who considered the hepatocyte vacuolation toxicologically insignificant. In my review of all the liver slides from the Bozo Research Center study, I noted that the vacuolation was minimal and variable in lobular distribution and chose to tabulate the lobular localization as diffuse. The hepatocellular vacuolation was minimal to mild and uniform across dose groups without a dose-response in severity. This hepatocyte cytoplasmic vacuolation affected a minority of the hepatic lobules and there was no associated hepatocellular cytotoxicity accompanying the vacuolation.
Occasionally other lesions common in rats of this age were present without any relationship to treatment (data not shown).
4. Discussion
4.1. Genotoxicity
The bacterial reverse mutation assay, two independent chromosome aberration assays, and an in vivo micronucleus test were conducted in compliance with Good Laboratory Practices at experienced laboratories. Ashitaba chalcone power (chalcone concentration 8%) in the bacterial reverse mutation assay with and without metabolic activation was not mutagenic. The two chromosome aberration assays of ashitaba powder (chalcone concentrations 8%) were without clastogenic effects at non-cytotoxic doses with or without metabolic activation. Following a range-finding study showing no sex difference in response, an in vivo micronucleus test was conducted at 2000 mg/kg ashitaba powder (chalcone concentration 7.2%) in male Swiss mice and did not alter the frequency of micronuclei in either polychromatic or normochromatic erythrocytes. Based on these findings ashitaba chalcone powder is considered non-mutagenic and without in vivo genotoxicity
4.2. Rat repeated dose toxicity study
4.2.1. Hematology
While a statistically significant reduced hemoglobin value was flagged in high dose males (Table 5a), related erythrocyte count and hematocrit values were within normal physiological range, indicating that this is a spurious statistical effect. The decreased platelet counts and increased coagulation indices in both sexes (Tables 5a and 5b) are a reflection of the known effects of Ashitaba chalcones on thrombane and are an expected pharmacological effect (Ko et al., 2004; Lin et al., 2001; Lo et al., 2009). The magnitude of the platelet decreases and PT and APTT increases is marginal and not of toxicological concern, especially in the absence of clinical signs, such as retinal hemorrhage, signifying a coagulation disorder (Walter et al., 2013). PT and APTT are relatively insensitive measures of coagulation and subject to variation due to technical issues associated with sample collection (Hall, 2013; Smith et al., 2013). Furthermore, fi- brinogen levels, which are rate limiting for coagulation, were similar among treated and control groups. In instances where APTT or PT are prolonged without an effect on fibrinogen and in the absence of liver failure, toxicologically significant effects on coagulation are not expected (Smith et al., 2013).
4.2.2. Clinical chemistry
Statistically significant increases were noted in serum alkaline phosphatase, total cholesterol, and phospholipid in high-dose (1000 mg/kg/day) male and female rats and elevated serum triglyceride in high-dose males (Tables 6a and 6b). Alkaline phosphatase (ALP) is a nonspecific, ubiquitous membrane associated enzyme with high levels in intestine, kidney, and bone. The contribution of the intestinal alkaline phosphatase isoenzyme is high in rats and the relative contribution of the alkaline phosphatase isoenzyme from bone is elevated in growing rats (Smith et al., 2013). In the absence of elevations of liver-specific alanine aminotransferase (ALT) or evidence of hepatocyte necrosis, the elevated alkaline phosphatase is not reflective of liver toxicity and its significance in this study is unknown. It is possible that the elevated alkaline phosphatase is of intestinal origin and related to the jejunal lymphangiectasia seen in high-dose males and females. An explanation for elevated aspartate aminotransferase (AST) in high-dose females is not apparent. AST is a cytosolic and mitochondrial enzyme that is not tissue specific. Small increases or decreases in serum cholesterol and triglyceride concentrations are common in non-clinical studies, may reflect minor alterations in lipid metabolism, and are typically nonadverse (Hall, 2013). The elevated serum levels of total cholesterol, triglyceride, and phospholipid are expected pharmacological effects of ashitaba chalcones that are known to increase cholesterol transport and circulating triglycerides (Nagata et al., 2007; Ogawa et al., 2003).
Other statistically flagged clinical chemistry analytes included blood urea nitrogen (BUN) and serum sodium and potassium in highdose males (Table 6a). The 12 mg/dL blood urea nitrogen in highdose males is within normal physiological limits, is not associated with elevations of serum creatinine or altered urinary measurements and may be related to the alpha 2-urinary globulin nephropathy in male rats (Table 7). Minor decreases in the serum electrolytes sodium and potassium seen in high-dose males are also within normal physiological limits. The small standard deviations typically associated with serum electrolyte measurements in rodent toxicity studies frequently yield spurious statistical flags and often reasons for small variations in serum electrolytes are not apparent (Smith et al., 2013), although they may reflect subtle homeostatic effects associated with minor differences in fluid balance and food consumptions that are not toxicologically significant (Hall, 2013). Minor shifts in some globulin isoforms seen in 300 and 1000 mg/ kg groups of males and females (Tables 6a and 6b) are not of toxicological concern in light of normal total protein, albumin and albumin/globulin ratios.
4.2.3. Histopathology
Potential test-article-related findings confirmed during poststudy independent histopathology peer review were adrenal cortical cytoplasmic vacuolation in 2 males and 3 females in the 1000 mg/ kg dose, dilated jejunal lacteals in both sexes at the 1000 mg/kg dose (Fig. 1) and a dose-related increased incidence of alpha 2-urinary globulin nephropathy in treated male rats (Table 7). The adrenal zona fasciculata cytoplasmic vacuolation is a common histological finding in rat toxicity studies (Laast et al., 2014) and in the absence of adrenal cortical cytotoxicity this change is non-adverse and unlikely a direct response to ashitaba chalcone powder.
The dilated jejunal lacteals in the high-dose male and female rats are consistent with lymphangiectasia, a change rarely seen in preclinical toxicity studies. Since intestinal lymphatics are responsible for transport of absorbed lipid, the occurrence of lymphangiectasia may be associated with the bolus administration of olive oil used as the vehicle for the test-article, although absence of lymphangiectasia in the other experimental groups that received olive oil suggests this change is not solely a response to the olive oil gavage. Lymphangiectasia has recently been reported in a preclinical rat study of indole 3-carbinol, a breakdown product in cruciferous vegetables with properties similar to ashitaba chalcones (Boyle et al., 2012). In that report the authors describe a potential mechanism possibly related to altered intestinal triglyceride resynthesis and chylomicron assembly. Since this change was limited to high-dose males and females and is rarely encountered in rodent toxicity studies, the NOAEL for this lesion is 300 mg/kg body weight.
A constellation of histological changes in the kidneys of treated male rats is well characterized as a male rat-specific alpha 2-urinary nephropathy (Frazier et al., 2012). Alpha 2-urinary globulin nephropathy is not regarded as significant to humans as it is a male rat-specific effect (Hard and Khan, 2004).
Two types of cytoplasmic vacuolation were reported in the livers of the rats. The fine periportal cytoplasmic vacuoles in control and treated rats are normally seen in preclinical rodent studies and are not toxicologically significant. The second type of cytoplasmic vacuolation consisting of larger cytoplasmic vacuoles was reported only in test-article-exposed rats and was more prominent in males than in females. In some rats and areas of the liver sections the vacuolation was more centrilobular while in other rats and areas of the liver sections the vacuolation was more diffusely distributed, involving some but not all liver lobules. The degree of vacuolation was minimal and morphologically consistent with intracytoplasmic triglyceride. The presence of minimal changes in serum lipid components in the clinical chemistry data is consistent with the presence of lipid droplets in hepatocytes. This type of change is typically reversible and consistent with fluctuations in lipid metabolism (Thoolen et al., 2010). In the absence of any indication of hepatocellular degeneration or necrosis, the lipid vacuolation is considered non-adverse at all dose levels.
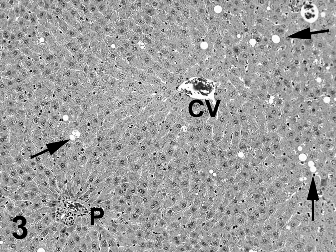
Fig. 3. Medium magnification of liver with diffusely scattered vacuoles (some identified by arrows) in hepatocytes from male rat #4001. Rat received 1000 mg/kg ashitaba chalcone for 90 days. This degree of vacuolation was graded as minimal. P = Portal triad. CV = Central Vein. Hematoxylin and eosin stain.
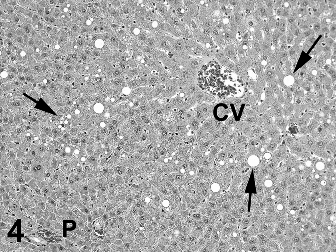
Fig. 4. Medium magnification of liver with diffusely scattered vacuoles (some identified by arrows) in hepatocytes from male rat #4006. Rat received 1000 mg/kg ashitaba chalcone for 90 days. This degree of vacuolation was graded as mild and represents the most severe example of hepatocyte vacuoles seen in the study. P = Portal triad. CV = Central Vein. Hematoxylin and eosin stain.
4.3. Human clinical exposure
In a 56-day double-blind, placebo-controlled study involving 26 slightly obese male and female adults, the treated group took a 200 mg capsule of ashitaba chalcone after dinner for 56 consecutive days. At the end of the study there were no differences between treated and placebo groups in visceral fat or body weight. However, compared to baseline, there was a significant reduction in visceral fat and body weight in the ashitaba group. In this study the daily intake of 200 mg of ashitaba chalcone for 56 days was considered safe. Protocol and full study details are available (Supplementary data).
4.4. Conclusions
Ashitaba chalcone has a long history of human use as a dietary supplement with claims of multiple heath benefits. Previously published animal studies were without toxic effects. Based on the genotoxicity and rat studies reported here, ashitaba chalcone powder is not genotoxic and is of general low toxicity with jejunal lymphangiectasia present in high dose (1000 mg/kg) males and females and a dose-related male rat-specific alpha 2-urinary globulin nephropathy in males. Based on the present findings, the NOAEL for ashitaba chalcone powder is 300 mg/kg in male and female rats.
Conflict of interest
Review of study materials and preparation of this manuscript was paid for by Japan BioScience Laboratory, Osaka, Japan. I served as a paid consultant for this work and have no financial or other interests in the sponsoring company or its products.
Transparency document
The Transparency document associated with this article can be found in the online version.
Acknowledgements
Preparation of this manuscript was paid for by Japan Bioscience Laboratory, Osaka, Japan.
Appendix: Supplementary material
Supplementary data to this article can be found online at doi:10.1016/j.fct.2014.12.021.
References
Boyle, M.C., Crabbs, T.A., Wyde, M.E., Painter, J.T., Hill, G.D., Malarkey, D.E., et al., 2012. Intestinal lymphangiectasis and lipidosis in rats following subchronic exposure to indole-3-carbinol via oral gavage. Toxicol. Pathol. 40, 561–576.
Chang, H.R., Lee, H.J., Ryu, J.H., 2014. Chalcones from Angelica keiskei attenuate the inflammatory responses by suppressing nuclear translocation of NF-kB. J. Med. Food 17, 1306–1313.
Dimmock, J.R., Elias, D.W., Beazely, M.A., Kandepu, N.M., 1999. Bioactivities of chalcones. Curr. Med. Chem. 6, 1125–1149.
Dorn, C., Bataille, F., Gaebele, E., Heilmann, J., Hellerbrand, C., 2010. Xanthohumol feeding does not impair organ function and homoeostasis in mice. Food Chem. Toxicol. 48, 1890–1897.
Enoki, T., Ohnogi, H., Nagamine, K., Kudo, Y., Sugiyama, K., Tanabe, M., et al., 2007. Antidiabetic activities of chalcones isolated from a Japanese Herb, Angelica keiskei. J. Agric. Food Chem. 55, 6013–6017.
Frazier, K.S., Seely, J.C., Hard, G.C., Betton, G., Burnett, R., Nakatsuji, S., et al., 2012. Proliferative and nonproliferative lesions of the rat and mouse urinary system. Toxicol. Pathol. 40, 14S–86S.
Hall, R., 2013. Principles of clinical pathology. In: Sahota, P., Popp, J., Hardisty, J., Gopinath, C. (Eds.), Toxicologic Pathology. Nonclinical Safety Assessment. CRC Press, Boca Raton, Florida, pp. 134–173.
Hard, G.C., Khan, K.N., 2004. A contemporary overview of chronic progressive nephropathy in the laboratory rat, and its significance for human risk assessment. Toxicol. Pathol. 32, 171–180.
Ko, H.H., Hsieh, H.K., Liu, C.T., Lin, H.C., Teng, C.M., Lin, C.N., 2004. Structure-activity relationship studies on chalcone derivatives: potent inhibition of platelet aggregation. J. Pharm. Pharmacol. 56, 1333–1337.
Laast, V.A., Larsen, T., Allison, N., Hoenerhoff, M.J., Boorman, G.A., 2014. Distinguishing cystic degeneration from other aging lesions in the adrenal cortex of SpragueDawley rats. Toxicol. Pathol. 42, 823–829.
Lin, C.-N., Hsieh, H.-K., Ko, H.-H., Hsu, M.-F., Lin, H.-C., Chang, Y.-L., et al., 2001. Chalcones as potent antiplatelet agents and calcium channel blockers. Drug Dev. Res. 53, 9–14.
Lo, H.M., Huang, T.F., Lin, C.N., Peng, H.C., Wu, W.B., 2009. 2’-Ethoxy-5’-methoxy-2- (5-methylthienyl)chalcone inhibits collagen-induced protein tyrosine phosphorylation and thromboxane formation during platelet aggregation and adhesion. Pharmacology 84, 145–152.
Nagata, J., Morino, T., Saito, M., 2007. Effects of dietary Angelica keiskei on serum and liver lipid profiles, and body fat accumulations in rats. J. Nutr. Sci. Vitaminol. (Tokyo) 53, 133–137
Nakamura, T., Tokushima, T., Kawabata, K., Yamamoto, N., Miyamoto, M., Ashida, H., 2012. Absorption and metabolism of 4-hydroxyderricin and xanthoangelol after oral administration of Angelica keiskei (Ashitaba) extract in mice. Arch. Biochem. Biophys. 521, 71–76.
Ogawa, H., Nakashima, S., Baba, K., 2003. Effects of dietary Angelica keiskei on lipid metabolism in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 30, 284–288.
Raj, C., Sarojini, B., Piyra, S., 2013. Protective efficacy of crude methanol extract of ashitaba green tea against radiation induced oxidative stress in E. coli K12 bacteria. Int. J. Nat. Prod. Res. 2, 39–43.
Seely, J.C., 2012. Histopathology Consultation Report on Selected Histopathological Findings Observed in a 13-Week Oral Toxicity Study of Ashitaba Chalcone Powder in Rats. Experimental Pathology Laboratory, Inc., Research Triangle Park, NC 27709, p. 92.
Smith, G., Walter, G., Walker, R., 2013. Clinical pathology in non-clinical toxicology testing. In: Haschek, W., Rousseaux, C., Wallig, M. (Eds.), Haschek and Rousseaux’s Handbook of Toxicologic Pathology. Academic Press, Amsterdam, pp. 565–594.
Thoolen, B., Maronpot, R.R., Harada, T., Nyska, A., Rousseaux, C., Nolte, T., et al., 2010. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol. Pathol. 38, 5S–81S.
Walter, G., Smith, G., Walker, R., 2013. Interpretation of clinical pathology results in non-clinical toxicology testing. In: Haschek, W., Rousseaux, C., Wallig, M. (Eds.), Haschek and Rousseaux’s Handbook of Toxicologic Pathology. Academic Press, Amsterdam, pp. 853–892.
Zhang, T., Sawada, K., Yamamoto, N., Ashida, H., 2013. 4-Hydroxyderricin and xanthoangelol from Ashitaba (Angelica keiskei) suppress differentiation of preadiopocytes to adipocytes via AMPK and MAPK pathways. Mol. Nutr. Food Res. 57, 1729–1740.