alpha-Glycosyl isoquercitrin (AGIQ) is highly absorbable and has been shown to possess antioxidative properties. Based on a favorable safety profile, it has been confirmed as generally recognized as safe (GRAS) compound by the FDA. Nevertheless, safety and toxicity information for AGIQ is still sparse. Therefore, the aim of this study was to test the safety and toxicokinetics of AGIQ in a 90-day study in 60 male and 60 female Sprague-Dawley rats at dietary doses up to 5%. All animals survived until scheduled euthanasia with no clinical signs of toxicity in any animal. AGIQ was rapidly absorbed with metabolism to quercetin and quercetin glucuronide at all dose levels. Statistically significant changes were noted in some tissue weights and clinical chemistry analytes, without evidence of systemic toxicity. The most prominent finding was systemic dose dependent yellow discoloration of bones of treated animals. However, no changes were observed microscopically, and this observation was concluded as toxicologically insignificant. The overall lack of adverse clinical signs, changes in body weight, feed consumption, clinical pathology parameters, and histopathological endpoints in animals administered AGIQ supports no observable adverse effect levels (NOAEL) of 5.0% in diet for both male and female rats (3461 mg/kg/day and 3867 mg/kg/day, respectively).
Keywords
- Flavonol;
- alpha-glycosyl isoquercitrin;
- Isoquercitrin;
- Quercetin;
- Toxicity;
- Safety;
- EMIQ;
- AGIQ;
- Sprague-Dawley;
- Toxicokinetics
Abbreviations
- AGIQ, alpha-glycosyl isoquercitrin;
- FEMA, flavor and extract manufacturers association;
- FOB, functional observation battery;
- GRAS, generally recognized as safe;
- ILS, Integrated Laboratory Systems;
- NBF, neutral buffered formalin
1. Introduction
Quercetin and its glycosides are natural flavonols present in many fruits and vegetables. Quercetin and rutin are poorly absorbed but glycosidic forms have enhanced bioavailability (Manach et al., 1997, Erlund et al., 2000, Day et al., 2001, Yang et al., 2005 and Hashimoto et al., 2006). Using rutin as a starting material, a highly glycosylated mixture can be produced from isoquercitrin (enzymatically decomposed rutin; quercetin-3-O–β D-glucoside) by transglycosylation with dextrin using cyclodextrin glucanotransferase to produce alpha-glycosyl isoquercitrin (AGIQ) ( Fig. 1), also known as enzymatically modified isoquercitrin (EMIQ) ( Manach et al., 1997 and Erlund et al., 2000).
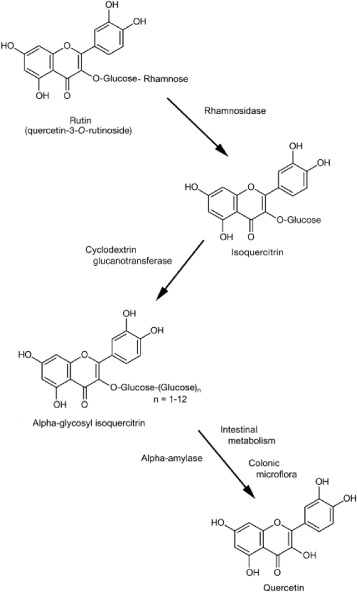
AGIQ is a mixture of isoquercitrins with one or more added glucose moieties (Akiyama et al., 2000 and FDA, 2007) (Fig. 2).
Upon ingestion, AGIQ is partially deglycosylated by salivary amylase, partially absorbed in the small intestine, and further metabolized by anaerobic enterobacteria in the large intestine with absorption of quercetin that is rapidly glucuronidated (Hollman et al., 1996,Day et al., 2001 and LePoole, 2003). Other large intestinal bacteria can act on the aromatic rings of quercetin to form short chain fatty acids and phenyl and hydroxyphenylacetic acids that are subsequently absorbed (Valentova et al., 2014).
Isoquercitrin (quercetin-3-O–β-D-glucoside) is a rare natural compound, which has attracted much attention in the food and pharmaceutical industries due to its long list of beneficial properties ( Wang et al., 2015). These include anti-inflammatory, hypotensive, anti-mutagenesis, anti-oxidative, anti-depressant, hypolipidemic and anti-viral effects (Amado et al., 2009, Gasparotto Junior et al., 2011, Kim et al., 2010, Li et al., 2011 and Valentova et al., 2014). Because of its linear glucose moieties, AGIQ is soluble in water, and is absorbed well when given orally (Hara et al., 2014). Similar to isoquercitrin, AGIQ has antioxidative ( Morita et al., 2011, Nishimura et al., 2010 and Shimada et al., 2010) and tumor suppressive properties ( Fujii et al., 2013,Hara et al., 2014 and Kimura et al., 2013) in experimental animals. When tested previously in rats, AGIQ was shown to be safe and non-carcinogenic in a number of toxicological studies ( Salim et al., 2004 and FDA, 2007).
AGIQ was developed in 1987 and approved by the Japanese Ministry of Health and Welfare for use as a food additive in 1996 (MHW, 2009, http://www.ffcr.or.jp/zaidan/FFCRHOME.nsf/pages/list-exst.add). Based on its favorable safety profile, AGIQ has been concluded by the Expert Panel of the Flavor and Extract Manufacturers Association (FEMA) (FEMA No. 4225) as a generally recognized as safe (GRAS) compound in 2005 (Smith et al., 2005). The U.S. Food and Drug Administration (US FDA) has granted a GRAS status for AGIQ as an anti-oxidant as well, based on the details given in the GRAS Notice (GRN 000220) (FDA, 2007). Nevertheless, when reviewing the available literature, it is evident that the safety and toxicity information for isoquercitrin and AGIQ is still sparse, and the published safety assessment reports are from older studies that are not Good Laboratory Practice (GLP)-compliant and/or that used AGIQ of low purity (Engen et al., 2015, Valentova et al., 2014 and Salim et al., 2004).
Two previously 13-week rat toxicity studies were conducted using enzymatically modified isoquercitrin (Tamano et al., 2001) and enzymatically decomposed rutin which is principally isoquercitrin (Hasumura et al., 2004). Neither study fully provided details of test agent purity. The Tamano study doses ranged from 0.3 to 2.5% in powdered diet and there was decreased body weight gain in both sexes at the highest dose. Other changes included increased urinary ketones in males at 2.5%, increased reticulocytes in higher doses of both sexes, and yellow pigmentation of bones. The authors suggested the NOAEL at 0.3% for both sexes of F344 rats. The highest dose in the Hasumura study was 5% and this dose was associated with a body weight gain decrement and decreased erythroid parameters in males. The authors concluded a NOAEL of 1% for males and 5% for female Wistar rats. A two-dose two-year study of enzymatically modified isoquercitrin in F344 rats was negative for carcinogenicity at 0.5% and 1.5% in the diet (Salim et al., 2004).
In anticipation of expanded marketing of AGIQ, this report is an initial part of a safety assessment of highly purified AGIQ, and includes a repeated dose 90-day study and a single dose toxicokinetic (TK) study in Sprague-Dawley rats in fully GLP-compliant studies.
2. Methods
2.1. Animal husbandry and maintenance
Previously published isoquercitrin rat studies were conducted in F344 and Wistar rats and diets were varied. We chose to use the Harlan Sprague-Dawley rat and Purina 5002 diet since that is our standard rat strain and diet for food additive studies and because our past use of this combination of rat strain and diet provides us a useful historical control database. Furthermore, in anticipation of our reproductive toxicity studies, we needed a rat strain with high fecundity. It is noted that the NTP recently switched from the F344 rat to the Sprague Dawley rat in part because the low fecundity of the F344 was not ideal for their reproductive toxicity studies.
Hsd: Sprague-Dawley rats, approximately 5 weeks old, were obtained from Harlan Laboratories. A Purina Certified 5002 Meal Diet (Ralston Purina Co., St. Louis, MO) was offered ad libitum throughout the study. The animals were allowed free access to drinking water, supplied to each cage via polycarbonate water bottles. All animals were housed singly in a polycarbonate cage with a micro-isolator top. Absorbent heat-treated hardwood bedding (Northeastern Products Corp., Warrensburg, NY) was provided and changed once per week. The rats were allowed a 7-day acclimation period to the facility conditions prior to inclusion in the study. The study was approved by the Integrated Laboratory Systems (ILS), Inc. (Research Triangle Park, NC, USA) Animal Care and Use Committee, all procedures were in compliance with the Animal Welfare Act Regulations (9 CFR 1–4), and animals were handled and treated according to the Guide for the Care and Use of Laboratory Animals ( ILAR, 2011).
2.2. Experimental design
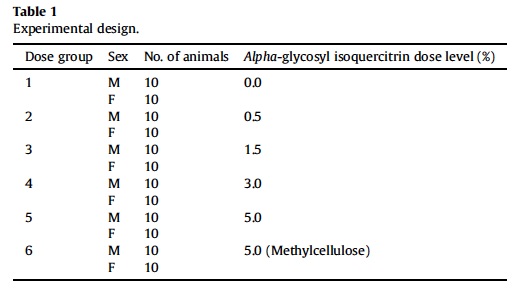
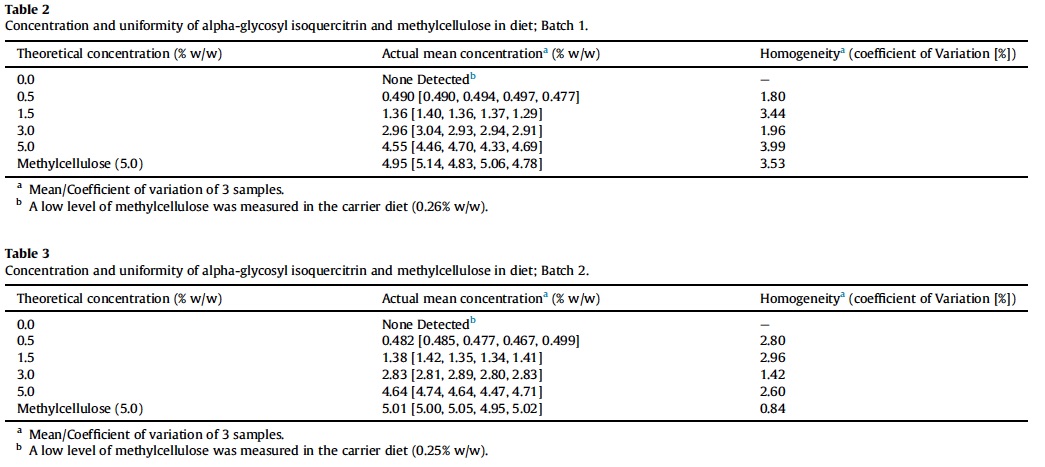
Ninety-day study was conducted in accordance with OECD Testing Guideline No. 408 (OECD, 1998). Sixty male and 60 female rats were randomized into six exposure groups (Table 1).
2.3. Viability, clinical signs, weight and food consumption
Each animal was observed for morbidity and mortality twice daily on weekdays and once daily on weekends/holidays, and cage-side observations were performed daily following initiation of exposure. Detailed clinical observations and body weight measurements were performed prior to exposure on Day 1, then weekly thereafter, and at termination. Feed consumption (g/kg body weight/day) was calculated weekly. Ophthalmological examinations were conducted in all rats prior to AGIQ or methylcellulose exposure. Within one week of study termination, control rats and those exposed to the high AGIQ dose were evaluated again. After 11 weeks of exposure, all animals were evaluated for their reactivity to auditory, visual and proprioceptive stimuli, and grip strength through a Functional Observational Battery (FOB) (OECD, 1997); motor activity was also assessed in which total activity was determined in 5-min intervals for an hour. For urine collection, animals were maintained in metabolic caging overnight for approximately 18-h provided with water ad libitum, but without access to feed. Urine was collected in collection cups surrounded by cold packs. Urine volume was measured and 15 mL, when available, was transferred for urinalysis.. Volume of urine, appearance, specific gravity, pH, glucose, protein, occult blood, ketones, bilirubin, urobilinogen, sodium, and potassium were recorded/evaluated and an examination of the sediment performed by microscopy.
2.4. Hematology, biochemistry and coagulation
Blood for hematology, biochemistry, and coagulation parameters, was collected from the vena cava at necropsy. Serum thyroid stimulating hormone, triiodothyronine (T3), and thyroxine (T4) levels were also determined.
2.5. Necropsy and tissue handling
Complete necropsies were performed on all animals following humane euthanasia at the end of the 3-month exposure period. At necropsy, all organs and tissues were examined for grossly visible lesions. Tissues were preserved in 10% neutral buffered formalin (NBF), except eyes and testes, which were fixed in Modified Davidson’s fluid and then preserved in NBF. Tissues for microscopic evaluation were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The following tissues were examined microscopically from male and/or female animals: gross lesions and tissue masses, adrenal gland, bone (sternum and femur) with marrow, aorta, brain, spinal cord, clitoral gland, esophagus, eyes, heart, large intestine (cecum, colon), small intestine (duodenum, jejunum, ileum), kidney, liver, lung, lymph nodes (mandibular and mesenteric), mammary gland, nose, ovary, oviduct, pancreas, parathyroid gland, pituitary gland, preputial gland, prostate gland, salivary gland, skin, skeletal muscle, sciatic nerve, spleen, stomach (forestomach and glandular), testis with epididymis and seminal vesicle, thymus, thyroid gland, tongue, trachea, urinary bladder, and uterus, cervix and vagina, Zymbal gland. The femur was examined in all treated groups. A semiquantitative grading scheme was used to evaluate the extent of the lesions in the tissue, generally using the criteria presented by Shackelford et al. (2002), using 5 grades, as follows: no lesion (grade 0), minimal (grade 1), mild (grade 2), moderate (grade 3), and marked (grade 4). Following completion of the studies, the accuracy of the histopathologic diagnosis was determined by a peer review, as outlined by the Society of Toxicologic Pathology (STP) (Morton et al., 2010).
2.6. Toxicokinetics study
A single dose TK study of AGIQ (>97% pure [with 0.1% quercetin]) was conducted in accordance with USFDA’s GLP regulations (21CFR Part 58) and was designed to satisfy OECD Testing Guideline No. 417: Toxicokinetics (OECD, 2010), specifically with respect to plasma/blood kinetics. This was a time-course study to determine estimates of basic TK parameters (Cmax, Tmax, area under the plasma concentration-time curve) at 1, 3, 6, 12, and 24 h following a single gavage dose of AGIQ. Twenty male Sprague Dawley rats were equally allocated to one of four designated groups at dose levels of 250, 500, 750 and 1000 mg/kg in a deionized water vehicle. The top dose was selected due to its low toxicity and as the limit dose as specified in the test guideline. Approximately 300 μL of blood were collected via the jugular vein catheter, discharged into an EDTA tube maintained at approximately 4 °C, and centrifuged at 1000 g for 10 min at 4 °C. Plasma was collected and discharged into a cryovial with 0.5 M ascorbic acid (10 μL per 50 μL of plasma) and stored at or below −70 °C until transfer for anlaysis for AGIQ, isoquercitrin, quercetin, and quercetin glucuronide using a validated Applied Biosystems API-3000 LC-MS/MS method (Applied Biosystems, Grand Island, NY, USA) at Alera Labs (Research Triangle Park, NC) with a lower limit of quantification of 20 ng/mL for all analytes. TK parameters were determined by Nuventra, Inc. (Durham, NC) using Phoenix WinNonlin® (Pharsight, St. Louis, MO) and R (R Foundation for Statistical Computing, Vienna, Austria) software. TK parameters for quercetin and quercetin glucuronide were derived using non-compartmental methods employing a validated installation of Pheonix WinNonlin® version 6.3 (Pharsight Corp, St. Louis, MO). The TK parameters were calculated by non-compartmental methods using individual animal plasma concentration-time data. The area under the concentration-time curve from zero-time (predose) to the time of last quantifiable concentration (AUClast) was calculated by a combination of linear and logarithmic trapezoidal methods. The linear trapezoidal method was employed for all incremental trapezoids arising from increasing concentrations and the logarithmic trapezoidal method was used for those arising from decreasing concentrations (linear up/log down method). Since all blood samples were taken at or near the scheduled time, nominal blood sampling times were used in the analysis.
2.7. Statistical analysis
Group mean and standard deviations were calculated and reported. All data from AGIQ-exposed animals were analyzed (final body weight, body weight gain, food consumption [g/kg/day], absolute and relative tissue weights, neurotoxicological endpoints, urinalysis endpoints, clinical pathology endpoints, and histopathological endpoints) using Statistical Analysis System version 9.2 (SAS Institute, Cary, NC). Data collected from methylcellulose animals were not analyzed as no statistically significant differences were observed in food consumption or body weights in animals exposed to AGIQ as compared to concurrent vehicle control animals. When an endpoint value was below the limit of quantification (i.e., T4), a value corresponding to half the limit of quantitation was assigned for the purpose of statistical analysis.
With the exception of neurotoxicological endpoints, studentized residual plots were used to detect possible outliers in the data. Homogeneity of variance was analyzed using Levene’s test. If the data were heterogeneous, then appropriate transformation (log, square root, multiplicative inverse) was performed and the data re-analyzed for homogeneity of variance. Data were then analyzed using a one-way analysis of variance and AGIQ exposed groups compared to the appropriate control group using Dunnett’s test. Heterogeneous data sets were analyzed using a Dunn’s test to compare exposed groups to the concurrent control group. Finally, dose-dependent changes were evaluated using a linear regression model.
Endpoints in the FOB using interval scales were evaluated for homogeneity using Levene’s Test of Homogeneity of Variances. A non-significant result (p > 0.001) indicated that an assumption of homogeneity of variance was appropriate, and the data were compared using the Analysis of Variance Test. The groups exposed to the test article were compared with the control group using Dunnett’s Test. If Levene’s Test was significant (p ≤ 0.001), the Kruskal-Wallis Test was used to analyze the data; in the event of a significant result (p ≤ 0.05), Dunn’s Test was used to compare the groups exposed to the test article with the control group.
Endpoints in the FOB using graded or count scales were analyzed using a non-parametric strategy. When 75% or fewer of the scores in all the groups were tied, the Kruskal-Wallis Test was used to analyze the data, and in the event of a significant result (p ≤ 0.05), Dunn’s Test was used to compare the groups exposed to the test article with the control group. When more than 75% of the scores in any group were tied, Fisher’s Exact Test was used to compare the proportion of ties in the groups. Endpoints in the FOB using descriptive or quantal scales were analyzed using the Fisher’s Exact Test.
Data from the motor activity test, with repeated measurements within a session, were analyzed using an Analysis of Variance with Repeated Measures. A significant effect (p ≤ 0.05) in that test can appear as effect of Dose (a difference between groups in the total across all measurements in a session) or as an interaction between Dose and Time (a difference between groups at specific measurement periods). If the Dose effect was significant, the totals for the control group and the groups administered the test article were compared using Dunnett’s Test. If the Dose × Time interaction was significant, a comparison of the groups using Dunnett’s Test was performed at each measurement period within the repeated measures model.
3. Results
3.1. Survival, clinical observations, and body and organ weight
All animals survived to the scheduled sacrifice with no animals showing signs of morbidity, and there were no AGIQ or methylcellulose-related abnormal clinical or cage-side observations noted during the course of the study. There were no significant changes in feed consumption, mean final body weight or body weight gain in AGIQ exposed rats when compared to concurrent controls. Feed and AGIQ consumption and mean final body weight and body weight gain are presented in Table 4.
3.2. Ophthalmoscopy
Examination of control and high dose group animals within a week of termination detected mild corneal crystals in two male rats in the control group and one male and one female rat in the high dose group. No other abnormalities were observed. Corneal crystals are a common finding in Sprague-Dawley rats and were not considered to have a causal relationship with AGIQ exposure (Taradach and Greaves, 1984).
3.3. Neurotoxicity screening
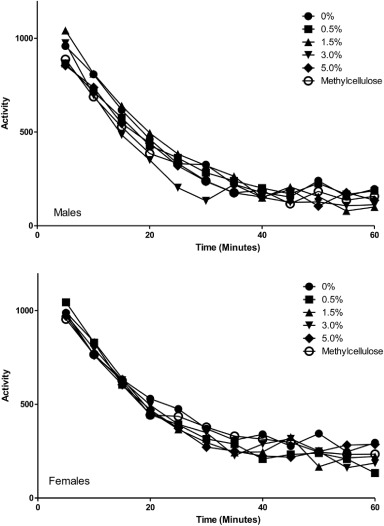
FOB evaluation and automated motor activity (Fig. 3) assessments of animals showed no significant changes in AGIQ exposed animals compared to concurrent controls.
3.4. Urinalysis
There were no significant changes in the urinalysis parameters (specific gravity, bilirubin, ketones, blood, pH, sodium, potassium, urobilinogen, and glucose) in male or female rats exposed to AGIQ as compared to the concurrent control groups. There was a significant decrease in total volume of urine from male rats exposed to 0.5% AGIQ compared to the concurrent control group. There was a statistically significant decreasing trend in urine protein in female, but not male rats, with no corresponding statistically positive dose groups. The noted changes are not of toxicologic concern due to not being reproduced in both genders and no statistically significant pair-wise comparisons in urine protein.
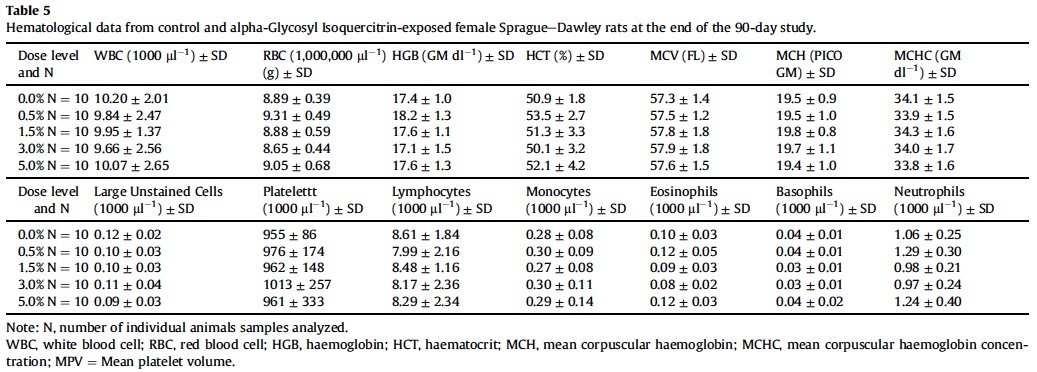
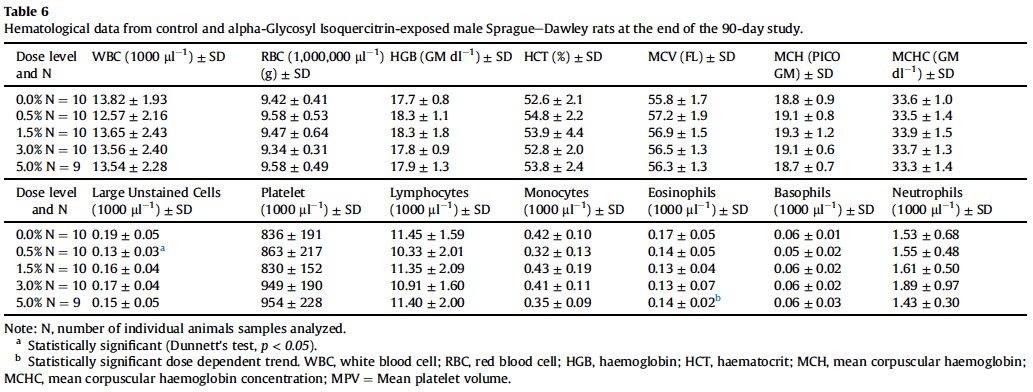
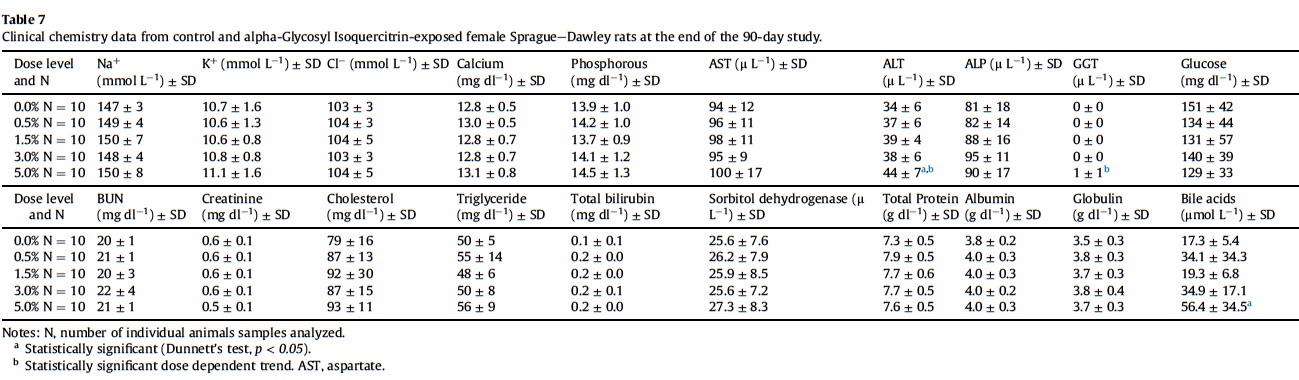
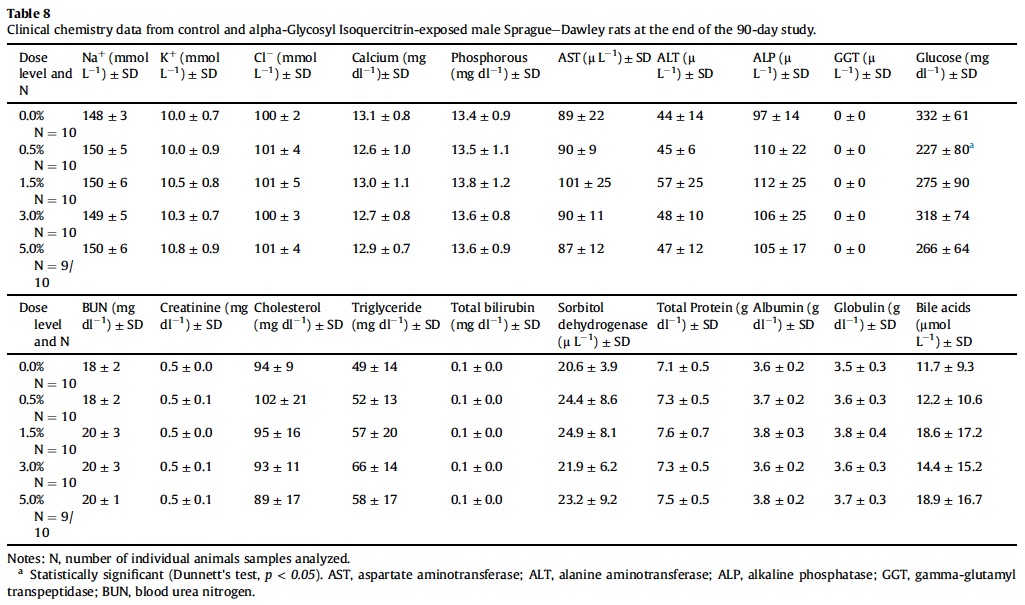
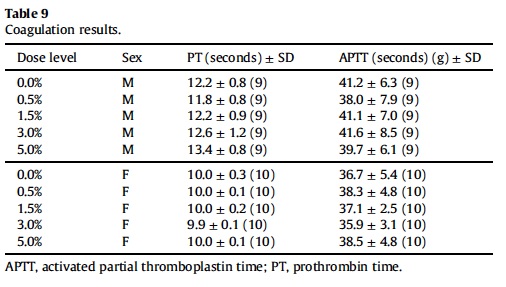
3.5. Hematology, coagulation and clinical chemistry
Mean hematological parameters, coagulation results, and clinical chemistry analysis data are presented in Table 5, Table 6, Table 7, Table 8 and Table 9. Statistically significant changes were noted in several coagulation, hematological, and clinical chemistry parameters. As is usual with high precision measurements, slight differences in mean values may be statistically different but not of toxicological concern. All of the statistical changes noted for hematology, coagulation and clinical chemistry are considered not related to treatment since there is no dose response for any of the statistically significant parameters; there are no related histopathological changes in any organ that may explain these changes; and all the statistical significant changes are within ILS historical data and the laboratory reference range for this strain and age of rats.
Levels of serum hormones T3, T4 and TSH were not significantly different from concurrent controls in either gender (data not shown).
3.6. Tissue weights
The majority of tissues examined showed no changes in their absolute and relative weights against body weight when compared to concurrent controls. Tissues with no weight changes in either gender compared to tissues of the concurrent control group included: brain, heart, lungs, salivary glands, spleen, thymus, thyroid, prostate, seminal vesicles, testes, and ovaries.
For males, a significant increase was observed in relative adrenal gland weight, absolute and relative epididymidal weights, absolute and relative kidney weights, absolute and relative liver weights and relative pituitary weights after exposure to AGIQ (Table 10). For females, a significant increase was seen in relative kidney and liver weights and absolute and relative uterine weights following exposure to AGIQ (Table 11). Absolute and relative pituitary weights were significantly decreased in female rats exposed to AGIQ compared to the concurrent control animals (Table 11).
The statistically significant changes in tissue weights for major metabolic organs were generally mild in nature, mostly fell within ILS’ historical control ranges, and often were not observed in the high dose group. Furthermore, the statistically significant changes were not associated with tissue-specific or relevant microscopic findings.
3.7. Macroscopic and microscopic observations
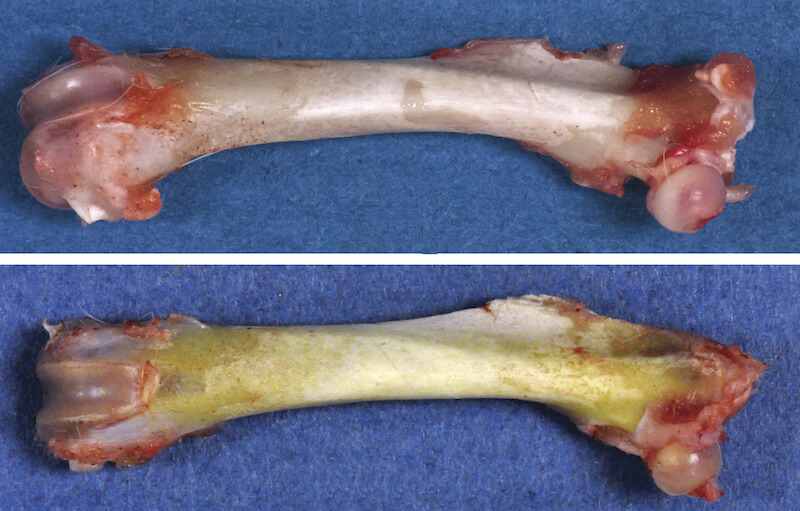
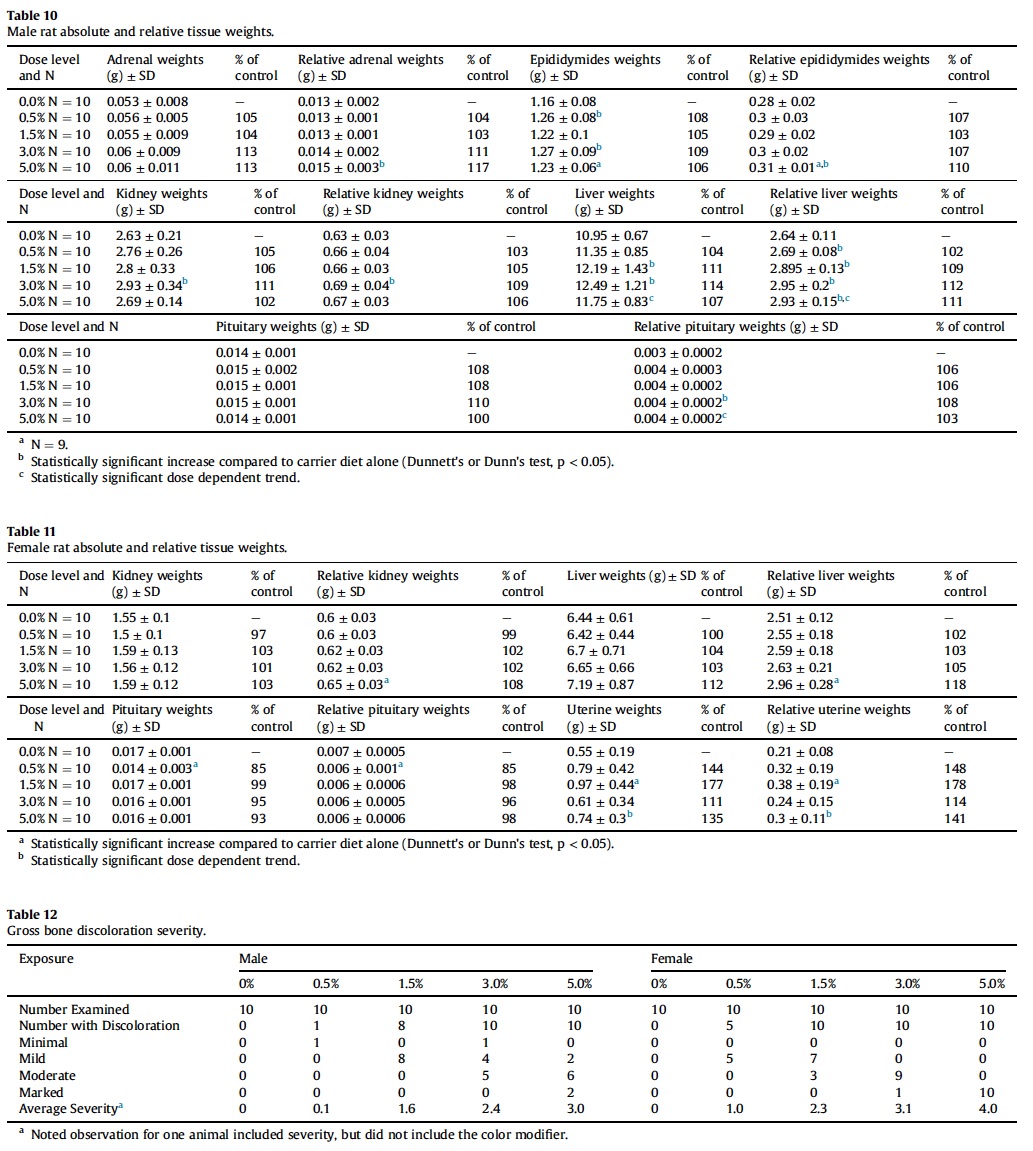
Diffuse yellow discoloration of all bones examined (i.e, femur, calvarium, maxilla), was noted grossly with increasing severity correlating with dose level (Table 12 and Fig. 4).
However, there were no microscopic changes in the femurs from any AGIQ exposed animals. No other significant macroscopic or microscopic observations were related to the AGIQ treatment. Background lesions commonly present in rats of the strain and age were present but without any exacerbation related to treatment (Table 13). In particular, in the male high dosed group, 8/10 cases of minimal dilation of the submucosal glands in the trachea were noted, in comparison to a single case seen in the control group. This change consisted of minimal dilation of the seromucous (combination of watery and mucoid secretion) glands normally found in the submucosa of the proximal portion of the rat trachea. This is a common background finding that is neither clinically nor toxicologically relevant.
3.8. Toxicokinetics
Plasma concentrations of monoglycosylated isoquercitrin, diglycosylated isoquercitrin, triglycosylated isoquercitrin, quercetin, and quercetin glucuronide were determined using a validate liquid chromatography/tandem mass spectrometry (LC-MS/MS) method with a 20 ng/mL lower limit of quantification for all analytes. Two samples were not obtaimed: a predose sample from one rat (500 mg/kg) and a 12-hour sample from one rat (1000 mg/kg). Missing samples had no notable impact on data interpretation, although it is possible that Tlast and AUClast for quercetin were underestimated; quercetin concentrations were quantifiable at 12 h postdose for 2 of the 4 other rats in the 1000 mg/kg dose group.
3.8.1. Monoglycosylated isoquercitrin, diglycosylated isoquercitrin and triglycosylated isoquercitrin
All results were below the limit of quantification.
3.8.2. Isoquercitrin
Isoquercitrin was measurable at 1 h postdose for all rats with the exception of one rat at 250 mg/kg and at 3 h postdose for only 1 rat at 750 mg/kg. Mean concentrations generally increased proportionally with increasing dose from 250 to 750 mg/kg, with mean values of 20.2 ng/mL, 38.6 ng/mL, and 55.0 ng/mL. However, the mean concentration at 1000 mg/kg (39.6 ng/mL) was similar to the mean concentration at 500 mg/kg. Inter-animal variability, assessed via the coefficient of variation (CV%) among samples collected at 1 h postdose, ranged from 16.1% to 58.5%.
3.8.3. Quercetin
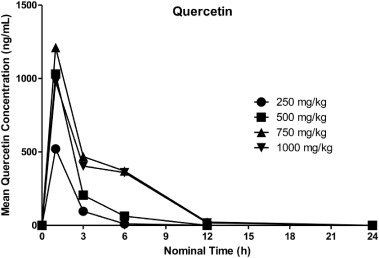
Quercetin was quantifiable (>20 ng/mL) from 1 h (the first timepoint) through 3 or 6 h postdose at 250 mg/kg, through 6 h postdose at 500 mg/kg, and through 6 or 12 h postdose at 750 and 1000 mg/kg. Mean concentrations increased as dose increased from 250 to 750 mg/kg, however, mean concentrations at 1000 mg/kg were slightly lower than those at 750 mg/kg. Mean quercetin concentrations peaked at 1 h postdose and then decreased through the last measurable timepoint, although concentrations decreased only slightly from 3 to 6 h postdose at 750 and 1000 mg/kg. Overall, inter-animal variability was moderate; where all values were quantifiable, CV% values calculated by dose group and timepoint ranged from 17.2% to 67.5%.
For individual rats, quercetin concentrations increased from 3 to 6 h postdose for 5 individuals (1 rat at 500 mg/kg and 2 each at 750 and 1000 mg/kg), and the maximum concentration was at 3 h postdose for one animal at 1000 mg/kg. The observed increase from 3 to 6 h postdose is suggestive of enterohepatic circulation, possibly through hydrolysis of the glucuronide metabolite by gut microbes and subsequent reabsorption of quercetin. Mean quercetin plasma concentration-time profiles following oral administration of AGIQ are presented in Fig. 5.
3.8.4. Quercetin glucuronide
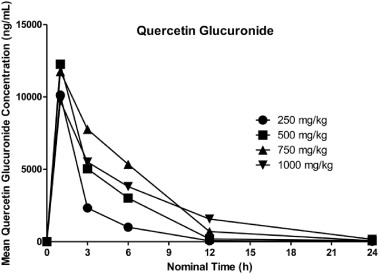
Quercetin glucuronide was quantifiable (>20 ng/mL) in rat plasma from 1 h through 24 h postdose, with an exception for 3 rats at 250 mg/kg and 1 rat at 750 mg/kg, where the last measurable sample was collected at 12 h postdose. Overall, mean concentrations generally increased with each increase in dose from 250 to 750 mg/kg, with an exception at 1 h postdose, where the value at 500 mg/kg was higher than at 750 mg/kg. Mean concentrations at 1000 mg/kg were lower than those at 750 mg/kg at 1, 3, and 6 h postdose, and higher thereafter. For all dose groups, mean concentrations decreased from 1 h postdose through 24 h postdose. Where all samples were quantifiable, CV% values by dose and timepoint ranged from 13.5% to 166%.
Individual profiles generally followed the mean, with exceptions for 1 or 2 animals per dose group, where concentrations increased (or were similar) from 3 to 6 h postdose or from 12 to 24 h postdose. Mean quercetin glucuronide plasma concentration-time profiles following oral administration of AGIQ are presented in Fig. 6.
3.8.5. Quercetin toxicokinetics
Key mean TK parameters for quercetin in the plasma of male rats are summarized in Table 14. All rats were systemically exposed to quercetin following oral (gavage) administration of AGIQ at doses of 250, 500, 750, and 1000 mg/kg. In terms of mean Cmax and AUClast, exposure increased with each increase in dose from 250 to 750 mg/kg and then decreased from 750 to 1000 mg/kg. Overall, a 3-fold increase in dose from 250 to 750 mg/kg resulted in a 4.7-fold increase in AUClast, a 3-fold increase in AUC0-6 and a 2.3-fold increase in Cmax. Quercetin exposure increased proportionally with the increase in dose from 250 to 500 mg/kg in terms of both Cmax and AUClast and from 250 to 750 mg/kg in terms of AUC0-6.
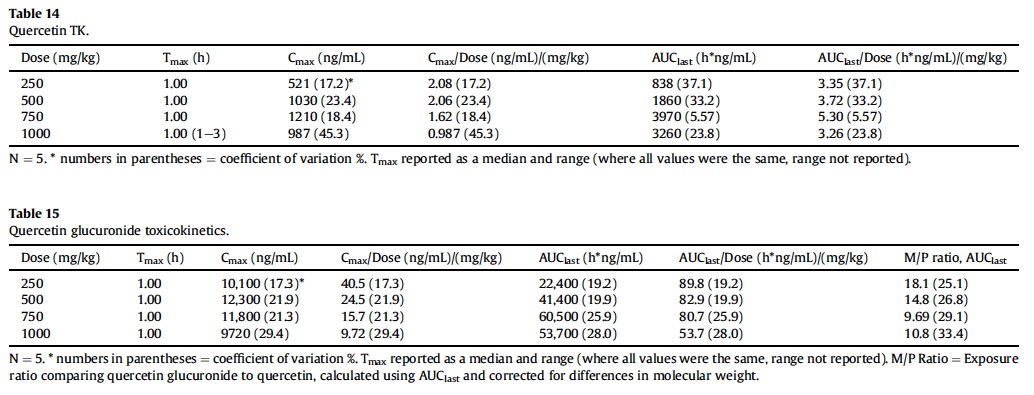
Tmax was 1 h postdose (the first timepoint) for all rats, with the exception of 1 rat at 1000 mg/kg, where it was 3 h postdose (and fairly similar at 1 and 3 h postdose for this rat). The terminal elimination phase was generally not apparent, precluding determination of rate dependent parameters (AUCinf and t½) for all but one rat at 750 mg/kg, where t½ was estimated to be 2.27 h.
3.8.6. Quercetin glucuronide toxicokinetics
Key mean TK parameters for plasma quercetin glucuronide are presented in Table 15.
All rats were systemically exposed to quercetin glucuronide following oral (gavage) administration of AGIQ at doses of 250, 500, 750, and 1000 mg/kg. For mean AUClast, exposure increased with each increase in dose from 250 to 750 mg/kg and then decreased from 750 to 1000 mg/kg. Mean Cmax was generally similar among dose groups, ranging from 9720 ng/mL at 1000 mg/kg to 12,300 ng/mL at 500 mg/kg. Mean AUClast increased proportionally with each increase in dose from 250 to 750 mg/kg while Cmax did not increase with increasing dose. Tmax was 1 h postdose (the first timepoint) for all rats. Terminal half-life (t½) was estimable for most animals and mean values ranged from 2.19 h (250 mg/kg) to 3.68 h (1000 mg/kg).
Exposure to quercetin glucuronide was greater than exposure to quercetin for all animals; mean ratios comparing AUClast ranged from 9.69 to 18.1 and the ratio generally decreased as dose increased. Mean ratios comparing Cmax ranged from 6.15 to 12.4 and also tended to decrease as dose increased.
4. Discussion
A main objective of the present study was to comprehensively evaluate the toxicity of AGIQ in Sprague-Dawley rats exposed orally for 90 days. The statistically significant changes in tissue weights and clinical pathology parameters that were observed following treatment did not indicate systemic toxicity. The changes were mild in nature, mostly fell within the historical control ranges, and often were not observed in the high dose group. Furthermore, the changes were not correlated with tissue relevant microscopic findings. Since there was an increase in relative liver weights (in males and females) coupled with an increase in alanine aminotransferase and bile acids levels (only in females) in animals exposed to the high dose, the liver histopathology was re-examined to confirm no corresponding microscopic changes in the liver (Fig. 7).
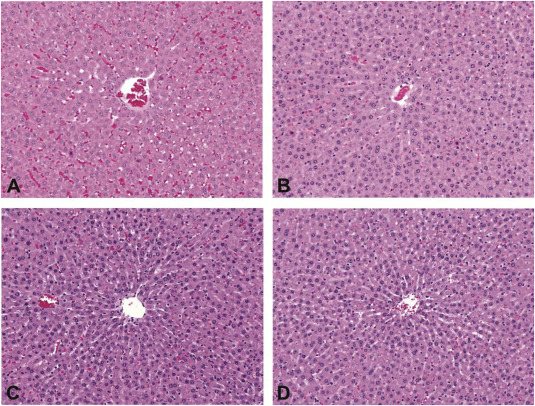
The National Toxicology Program (NTP) previously reported renal lesions in male rats exposed to quercetin, including increased severity of chronic nephropathy, hyperplasia and neoplasia of the renal tubular epithelium in a 2-year F344 rat study (Dunnick and Hailey, 1992). The chronic nephropathy mode of action represents a secondary mechanism for renal tumor development unique to the rat and has no relevance for extrapolation to humans (Hard et al., 2007). It is noted that in the present study, AGIQ exposure was not associated with an exacerbation of chronic nephropathy and kidney weight changes were not associated with any other potential exposure-related renal lesions.
The only notable macroscopic finding during this study was a dose-dependent yellow discoloration of bones, which was most prominent in the femurs. In a previous 13-week oral toxicity and 4-week recovery study with AGIQ in F344/DuCri Rats, yellow discoloration of bone was also evident, again without accompanying histological changes (Tamano et al., 2001). There was no indication for altered bone growth in any of the treatment groups, indicating that there are no adverse effects on this tissue. Since bones are dynamic tissues that are rapidly growing and constantly remodeling in rats of this age, the present study is of sufficient duration to have allowed detection of histopathological changes. Additionally, there were no serum clinical chemistry alterations in calcium or inorganic phosphate that would indicate a perturbation of bone metabolism. Taken together, we conclude that the bone discoloration is not clinically meaningful.
AGIQ is supplied as a yellow to yellow-orange powder. Therefore, it is most likely that the mechanism for the observed yellowish pigmentation of the bones is the deposition of yellow pigment, in a similar manner to carotenodermia. Carotenodermia is the yellowish discoloration of the skin due to yellow pigment deposition in the subcutaneous tissue, which can be seen after consumption of carotene-containing foods such as carrots and squash (Chiriac et al., 2014) or other yellow-orange compounds such as curcumin (Horev et al., 2015). Carotenodermia is a harmless and reversible condition, and according to the food and agriculture organization of the United Nations and the World Health Organization joint committee on food additives is not considered to be an adverse event (JECFA, 2015).
Toxicokinetic results clearly indicate absorption of AGIQ with rapid metabolic breakdown yielding plasma concentrations of 20 ng/mL or greater of quercetin and quercetin glucuronide at all dose levels. Transient plasma levels of isoquercitrin were detected primarily at 1-h post dose with plasma levels of AGIQ below the level of quantitation. Fluctuations observed for several individual animals around 6 h postdose are suggestive of enterohepatic circulation. Exposure to quercetin glucuronide was greater than exposure to quercetin for all animals; mean exposure ratios ranged from 9.69 to 18.1 for AUClast and from 6.15 to 12.4 for Cmax. Ratios of quercetin glucuronide relative to quercetin tended to decrease as dose increased.
Toxicokinetic evaluation of exposed male rats clearly demonstrated absorption of AGIQ at 250 mg/kg and greater with rapid metabolism yielding plasma levels of quercetin and quercetin glucuronide. The overall lack of adverse clinical signs, changes in body weight, feed consumption, clinical pathology parameters, and histopathological endpoints in animals administered AGIQ indicates a no observable adverse effect levels (NOAEL) of 5.0% in diet for both male and female rats (3461 mg/kg/day and 3867 mg/kg/day, respectively).
Conflict of interest
No potential conflict of interest was reported by the authors.
Funding disclosure
Integrated Laboratory Systems, Inc., was responsible for the study design, collection, analysis and interpretation of data and for the preparation of the final study report. The manuscript was prepared by A. Nyska. The decision to submit the paper for publication was made by San-Ei Gen, F.F.I., Inc.
References
Akiyama et al., 2000
T. Akiyama, T. Washino, T. Yamada, T. Koda, T. Maitani
Constituents of enzymatically modified isoquercitrin and enzymatically modified rutin (extract)
J. Food Hyg. Soc. Jpn., 41 (2000), pp. 46–60
Amado et al., 2009
N.G. Amado, D.M. Cerqueira, F.S. Menezes, J.F. da Silva, V.M. Neto, J.G. Abreu
Isoquercitrin isolated from Hyptis fasciculata reduces glioblastoma cell proliferation and changes beta-catenin cellular localization
Anticancer. Drugs, 20 (2009), pp. 543–552
Chiriac et al., 2014
A. Chiriac, A.E. Chiriac, T. Pinteala, E. Gologan, C. Solovan, P. Brzezinski
Yellow palms and feet in a child-case report and review
Russ. OMJ, 3 (2014)
Day et al., 2001
A.J. Day, F. Mellon, D. Barron, G. Sarrazin, M.R.A. Morgan, G. Williamson
Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin
Free Radic. Res., 35 (2001), pp. 941–952
Dunnick and Hailey, 1992
J.K. Dunnick, J.R. Hailey
Toxicity and carcinogenicity studies of quercetin, a natural component of foods
Fundam. Appl. Toxicol., 19 (1992), pp. 423–431
Engen et al., 2015
A. Engen, J. Maeda, D.E. Wozniak, C.A. Brents, J.J. Bell, M. Uesaka, Y. Aizawa, T.A. Kato
Induction of cytotoxic and genotoxic responses by natural and novel quercetin glycosides
Mutat. Res. Genet. Toxicol. Environ. Mutagen, 784–785 (2015), pp. 15–22
Erlund et al., 2000
I. Erlund, T. Kosonen, G. Alfthan, J. Maenpaa, K. Perttunen, J. Kenraali, J. Parantainen, A. Aro
Pharmacokinetics of quercitin from quercitin aglycone and rutin in healthy volunteers
Eur. J. Clin. Pharmacol., 56 (2000), pp. 545–553
FDA, 2007
U. FDA
Agency Response Letter GRAS Notice No. GRN00220 [Alpha-glycosyl Isoquercitrin]
US Food and Drug Administration Center for Food Safety and Applied Nutrition (2007)
Fujii et al., 2013
Y. Fujii, M. Kimura, Y. Ishii, R. Yamamoto, R. Morita, S.M. Hayashi, K. Suzuki, M. Shibutani
Effect of enzymatically modified isoquercitrin on preneoplastic liver cell lesions induced by thioacetamide promotion in a two-stage hepatocarcinogenesis model using rats
Toxicology, 305 (2013), pp. 30–40
Gasparotto Junior et al., 2011
A. Gasparotto Junior, F.M. Gasparotto, E.L. Lourenco, S. Crestani, M.E. Stefanello, M.J. Salvador, J.E. da Silva-Santos, M.C. Marques, C.A. Kassuya
Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: evidence for the inhibition of angiotensin converting enzyme
J. Ethnopharmacol., 134 (2011), pp. 363–372
Hara et al., 2014
S. Hara, R. Morita, T. Ogawa, R. Segawa, N. Takimoto, K. Suzuki, N. Hamadate, S.M. Hayashi, A. Odachi, I. Ogiwara, S. Shibusawa, T. Yoshida, M. Shibutani
Tumor suppression effects of bilberry extracts and enzymatically modified isoquercitrin in early preneoplastic liver cell lesions induced by piperonyl butoxide promotion in a two-stage rat hepatocarcinogenesis model
Exp. Toxicol. Pathol., 66 (2014), pp. 225–234
Hard et al., 2007
G.C. Hard, J.C. Seely, L.J. Betz, S.M. Hayashi
Re-evaluation of the kidney tumors and renal histopathology occurring in a 2-year rat carcinogenicity bioassay of quercetin
Food Chem. Toxicol., 45 (2007), pp. 600–608
Hashimoto et al., 2006
T. Hashimoto, Y. Ueda, N. Ol, H. Sakakibara, C. Piao, H. Ashida, M. Goto, K. Kanazawa
Effects of combined administration of quercetin, rutin, and extract of white radish sprout rich in kaempferol glycosides on the metabolism in rats
Biosci. Biotechnol. Biochem., 70 (2006), pp. 279–281
Hasumura et al., 2004
M. Hasumura, K. Yasuhara, T. Tamura, T. Imai, K. Mitsumori, M. Hirose
Evaluation of the toxicity of enzymatically decomposed rutin with 13- weeks dietary administration to Wistar rats
Food Chem. Toxicol., 42 (2004), pp. 439–444
Hollman et al., 1996
P.C.H. Hollman, M. van der Gaag, M.J.B. Mengelers, J.M.P. van Trup, J.H.M. de Vries, M.B. Katan
Absorption and disposition kinetics of the dietray antioxidant quercetin in man
Free Radic. Biol. Med., 21 (1996), pp. 703–707
Horev et al., 2015
L. Horev, Y. Ramot, L. Klapholz
Yellow feet in a patient with breast and thyroid carcinoma, due to oral intake of turmeric
Drug Saf. – Case Rep., 2 (2015)
ILAR, 2011
ILAR
Guide for the Care and Use of Laboratory Animals
220 pp. (eighth ed.)The National Academies Press, Washington (DC) (2011)
JECFA., 2015
JECFA.
Safety Evaluation of Certain Food Additives
WHO Food Addict. Ser., 70 (2015)
Kim et al., 2010
Y. Kim, S. Narayanan, K.O. Chang
Inhibition of influenza virus replication by plant-derived isoquercetin
Antivir. Res., 88 (2010), pp. 227–235
Kimura et al., 2013
M. Kimura, Y. Fujii, R. Yamamoto, A. Yafune, S.M. Hayashi, K. Suzuki, M. Shibutani
Involvement of multiple cell cycle aberrations in early preneoplastic liver cell lesions by tumor promotion with thioacetamide in a two-stage rat hepatocarcinogenesis model
Exp. Toxicol. Pathol., 65 (2013), pp. 979–988
LePoole, 2003
H. LePoole
Bioavailability and pharmacokinetics of quercetin aglycone and glycosides rutin and isoquercitrin
NutraCos (2003) Nov/Dec, 6–8
Li et al., 2011
R. Li, C. Yuan, C. Dong, S. Shuang, M.M. Choi
In vivo antioxidative effect of isoquercitrin on cadmium-induced oxidative damage to mouse liver and kidney
Naunyn. Schmiedeb. Arch. Pharmacol., 383 (2011), pp. 437–445
Manach et al., 1997
C. Manach, C. Morand, C. Demigne, O. Texier, F. Regerat, C. Remesy
Bioavailability of rutin and quercitin in rats
FEBS Lett., 409 (1997), pp. 12–16
Ministry of Health and Welfare, 2009
Ministry of Health and Welfare
List of Existing Food Additives
(2009) http://www.ffcr.or.jp/zaidan/FFCRHOME.nsf/pages/list-exst.add
Morita et al., 2011
R. Morita, K. Shimamoto, Y. Ishii, K. Kuwata, B. Ogawa, M. Imaoka, S.M. Hayashi, K. Suzuki, M. Shibutani, K. Mitsumori
Suppressive effect of enzymatically modified isoquercitrin on phenobarbital-induced liver tumor promotion in rats
Arch. Toxicol., 85 (2011), pp. 1475–1484
Morton et al., 2010
D. Morton, R.S. Sellers, E. Barale-Thomas, B. Bolon, C. George, J.F. Hardisty, A. Irizarry, J.S. McKay, M. Odin, M. Teranishi
Recommendations for pathology peer review
Toxicol. Pathol., 38 (2010), pp. 1118–1127
Nishimura et al., 2010
J. Nishimura, Y. Saegusa, Y. Dewa, M. Jin, M. Kawai, S. Kemmochi, T. Harada, S.M. Hayashi, M. Shibutani, K. Mitsumori
Antioxidant enzymatically modified isoquercitrin or melatonin supplementation reduces oxidative stress-mediated hepatocellular tumor promotion of oxfendazole in rats
Arch. Toxicol., 84 (2010), pp. 143–153
OECD, 1997
OECD, Test guideline 424
OECD Guideline for Testing of Chemicals, Neurotoxicity Study in Rodents, OECD
France, Paris (1997)
OECD, 1998
OECD
OECD Guideline for the Testing of Chemicals. No. 408. Repeated Dose 90-day Oral Toxicity Study in Rodents
10pp OECD, Paris, France (1998)
Salim et al., 2004
E.I. Salim, M. Kaneko, H. Wanibuchi, K. Morimura, S. Fukushima
Lack of carcinogenicity of enzymatically modified isoquercitrin in F344/DuCrj rats
Food Chem. Toxicol., 42 (2004), pp. 1949–1969
Shackelford et al., 2002
C. Shackelford, G. Long, J. Wolf, C. Okerberg, R. Herbert
Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies
Toxicol. Pathol., 30 (2002), pp. 93–96
Shimada et al., 2010
Y. Shimada, Y. Dewa, R. Ichimura, T. Suzuki, S. Mizukami, S.M. Hayashi, M. Shibutani, K. Mitsumori
Antioxidant enzymatically modified isoquercitrin suppresses the development of liver preneoplastic lesions in rats induced by beta-naphthoflavone
Toxicology, 268 (2010), pp. 213–218
Smith et al., 2005
R.L. Smith, S.M. Cohen, J. Doull, V.J. Feron, J.I. Goodman, L.J. Marnett, I.C. Munro, P.S. Portoghese, W.J. Waddell, B.M. Wagner, T.B. Adams, Expert Panel of the F, Extract Manufacturers A
Criteria for the safety evaluation of flavoring substances. The expert panel of the flavor and extract manufacturers association
Food Chem. Toxicol., 43 (2005), pp. 1141–1177
Tamano et al., 2001
S. Tamano, Y. Hatahara, M. Sano, A. Hagiwara, M. Nakamura, T. Wahino, K. Imaida
13-Week oral toxicity and 4-week recovery study of enzymatically modified isoquercitrin in F344/DuCrj rats
Jpn. J. Food Chem., 8 (2001), pp. 161–167
Taradach and Greaves, 1984
C. Taradach, P. Greaves
Spontaneous eye lesions in laboratory animals: incidence in relation to age
Crit. Rev. Toxicol., 12 (2) (1984), pp. 121–147
Teo et al., 2001
S.K. Teo, M.G. Evans, M.J. Brockman, J. Ehrhart, J.M. Morgan, D.I. Stirling, S.D. Thomas
Safety profile of thalidomide after 53 weeks of oral administration in beagle dogs
Toxicol. Sci., 59 (2001), pp. 160–168
Valentova et al., 2014
K. Valentova, J. Vrba, M. Bancirova, J. Ulrichova, V. Kren
Isoquercitrin: pharmacology, toxicology, and metabolism
Food Chem. Toxicol., 68 (2014), pp. 267–282
Wang et al., 2015
J. Wang, A. Gong, C.F. Yang, Q. Bao, X.Y. Shi, B.B. Han, X.Y. Wu, F.A. Wu
An effective biphase system accelerates hesperidinase-catalyzed conversion of rutin to isoquercitrin
Sci. Rep., 5 (2015), p. 8682
Yang et al., 2005
C.Y. Yang, S.L. Hsiu, K.C. Wen, S.P. Lin, S.Y. Tsai, Y.C. Hou, P.D. Lee Chao
Bioavailability and metabolic pharmacokinetics of rutin and quercitin in rats
J. Food. Drug. Anal., 13 (2005), pp. 244–250