Gabriel Siegel, Dan Regelman, Robert Maronpot, Moti Rosenstock, and Abraham Nyska
View as PDFAbstract: Real-time telepathology for use in investigative and regulated preclinical toxicology studies is now feasible. Newly developed microscope-integrated telepathology systems enable geographically remote stakeholders to view the live histopathology slide as seen by the study pathologist within the microscope. Simultaneous online viewing and dialog between study pathologist and remote colleagues is an efficient and cost-effective means for consultation, pathology working groups, and peer review, facilitating good science and economic benefits by enabling more timely and informed clinical decisions.
Preclinical studies in development of drugs and new treatment modalities are lengthy and resource-intense endeavors. Outsourcing these studies to contract research organizations (CROs) is increasingly common and often involves third-party toxicologic pathologists for study evaluation and pathology peer review. Considering the cost of running these studies, as well as their importance for public health and vetting new treatment modalities, it is imperative that the pathology evaluation following the completion of the in-life phase of the preclinical evaluation be conducted in an efficient manner that permits early and continued participation of all stakeholders.
The toxicologic pathology evaluation of investigative and preclinical studies is ideally performed by multiple pathologists sharing a multiheaded microscope. Yet pathology peer review can be successfully performed at a site remote from the study pathologist (Morton et al. 2010) so long as the peerreview pathologist can effectively evaluate tissue changes using the images of each entire specimen represented on the glass slides, while maintaining the image quality, workflow efficiency, and peer interaction that is comparable or better than using a multiheaded microscope located at the same site. As such, pathology peer review, consultations, and pathology working groups can become more efficient with simpler logistics by enabling parties not located by the microscope (i.e., peer-review pathologists, specific target tissue pathology experts, study toxicologists, sponsors, CRO study directors, etc.) to view, consult, and discuss specific histological changes with the other pathologist simultaneously viewing these slides under the microscope. Furthermore, as most study costs are either directly or indirectly borne by the sponsor, the ability to share slides with remote parties brings economic benefits via a reduction in travel and shipping, while enabling business and research and development decisions to be made faster and with greater clinical insight.
The Current State of Telepathology
“Telepathology is a form of communication where digital pathology images and accompanying clinical information are transmitted between medical professionals for clinical purposes including intraoperative consultations/frozen sections, second opinion consultations, primary diagnosis and quality assurance (Hanna, Pantanowitz, and Evans 2015). Pathology departments are increasingly looking to implement different digital pathology platforms, including whole slide imaging (WSI) systems, for a broad range of applications in patient care.”
Telepathology has traditionally been associated with WSI, a technology that creates a permanent digital copy of the glass slide at multiple magnifications and enables distant parties to view the information, select image fields for photography, and collect in-depth image analysis data in addition to simple measurements. Along with the clear advantages of WSI for creating digital data from glass slides, when attempting to utilize it for real-time telepathology applications, it is found that the time required for scanning slides is high, and the digitally heavy files containing multiple magnifications are not easily transferred to remote parties.
Furthermore, WSI typically represents a single flat plane of focus,while the eventual viewing of the file by the remote party is generally done as a single user and not as a simultaneous dynamic discussion involving the study pathologist and multiple stakeholders. WSI is also associated with a high capital expense with ongoing maintenance costs.All the above substantially reduce the utility and effectiveness of WSI when compared to a physically colocated discussion using a multiheaded microscope.
Since the pathologist’s primary tool is still the microscope, an effective means for instant and interactive sharing of a high-quality live view of the microscope’s optical field is the basic requirement for any telepathology application.
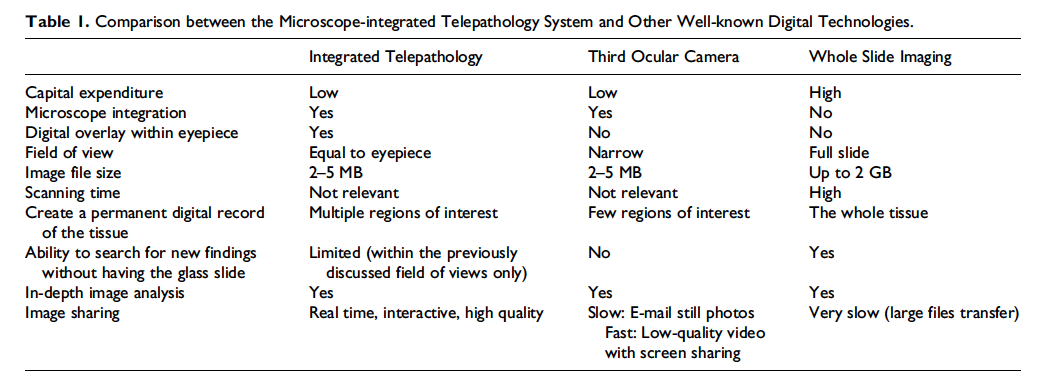
Indeed, scientific cameras installed in the third ocular of the microscope have been connected to screen-sharing applications for many years, successfully portraying a live view of the tissue in multiple focal planes and magnifications. However, the optical design of third ocular cameras captures only a narrow fraction of the field of view as seen by the microscope user to be viewed by distant parties (the field of view for third ocular cameras is generally around 35%, depending on the camera sensor size). Optical fields equivalent to the view from the microscope eyepiece are essential for discussion between the parties, since the surrounding tissue might be of importance along with the intentionally centered main region of interest. Furthermore, consumer-oriented screen-sharing applications substitute image quality for speed, leaving the remote viewer with compressed images that are not of digital pathology grade, do not enable annotations and morphometric calculations, nor create an atmosphere of multidirectional collaboration. For these reasons, third ocular scientific cameras do not lend well to real-time telepathology (Table 1).
The Introduction of Microscope-Integrated Telepathology
With the introduction of microscope-integrated telepathology systems, today there is the technology to share a high-quality, comparable, and live view of the microscope’s optical plane with remote parties. Such a technology enables multidirectional collaboration, sharing the microscope optical field on one side and high-quality full-resolution uncompressed analysis grade digital images on one or more remote sides.
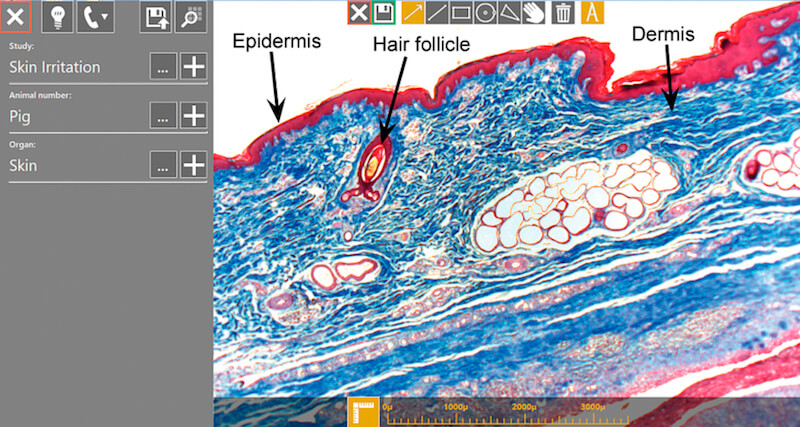
The method in which the new telepathology technology works is depicted in Figure 1. A small optical module becomes an integrated component of the study pathologist’s existing microscope by being placed in the optical path above the nosepiece and below the eyepieces. The microscope user continues to view the tissue through the eyepiece, while an embedded camera within the optical module captures a live feed of the tissue. Without the use of scanning, nor requiring any additional time on the part of the user, the optical module enables remote parties to view and discuss a live image that is of high digital pathology grade and similar to the dimensions of the tissue as seen within the microscope eyepiece.
The optical module is connected to a computer with an Internet connection and can be placed on any infinity-corrected microscope from all manufacturers. A built-in dedicated communication protocol overcomes the challenge of Internet latency, enabling all remote users to view the same full-quality digital pathology grade image regardless of individual connection speed.
The optical module further enhances pathology workflow by projecting an augmented overlay of digital information on top of the optical field of view within the microscope eyepiece. The microscope user may perform annotations, morphometric calculations, or compare digital images to the existing slide, such as making animal to animal comparisons during the slide evaluation phase, while all the time looking within the microscope eyepiece. All those actions are automatically shared and visible by all remote participants. The microscope user may optionally work on an adjacent computer screen attached to the computer and see exactly the same view as all other group participants.
During a telepathology session, a theoretically unlimited number of remote viewers may connect to the user’s microscope by downloading a client software that is configured to connect to the specific optical module. The remote viewers talk to the microscope user over their regular telephone or any other digital voice communication means, while at the same time viewing the live microscope field on the computer screen. During the telepathology session, all remote viewers may perform annotations, morphometric calculations, and download images directly to their personal computer,while the microscope user can work normally while sharing the tissue features using the mechanical stage and change objectives for increased magnification as necessary.
Via the optical module, any annotations performed by the remote viewers on their computer screens are viewed by the microscope user in real time as an augmented projection on top of the tissue within the microscope eyepiece (Figure 2). Of significant importance, the telepathology session can be performed over a secure point-to-point Internet connection without storing the data on any outside server, effectively limiting the ability of uninvited parties to view the telepathology session. General security measures are, however, encouraged for any use of Internet-connected personal computers.
As the in-depth, multidirectional collaboration between the microscope user and remote viewers is the desired goal, the new telepathology system allows both the microscope user and remote viewers to securely engage in active discussion while viewing a high-grade digital pathology image with a nearly identical view of the tissue dimensions—all without any change or distortion of the regular optical view. After a brief instruction and training, toxicologic pathologists, sponsors, study directors, and project managers can collaborate in real time.
Applications of Telepathology in Toxicologic Pathology
Among the principle applications of real-time telepathology within toxicologic pathology are consultations among pathologists and sponsors, online peer review, and group consultations as part of investigative studies, science advisory boards, and pathology working groups.
With respect to peer review, the intention is to ensure that treatment-related findings are properly identified, consistently diagnosed, and correctly interpreted (Crissman et al. 2004), and the use of telepathology systems as described herewill facilitate that intention.
As an example application of pathology peer review, an online peer review was recently conducted, involving a number of the authors using the abovementioned microscope-integrated telepathology system. The peer-review pathologist located in Timrat, Israel, received approximately 900 study slides from the study pathologist located at a U.S.-based CRO who was performing a preclinical study on behalf of a non-U.S. pharmaceutical corporation. After initial review, the peer-review pathologist set aside 11 of the 900 slides for further review due to a difference in opinion regarding the classification of the lesions. The online peer review included the peer-review pathologist, study pathologist, study director, and a consultant pathologist representing the corporate study sponsor. The session lasted 1 hr and ended in a consensus regarding all 11 slides.
In respect to pathology working groups, it is accepted that the cost and logistics of bringing together 5 pathologists are high. The issue at stake is generally the life of the compound and thereby requires utmost expertise and professionalism. We fully acknowledge the advantages of face-to-face discussions in order to reach the consensus, yet we believe the new technology may enable online working groups to be conducted with a significant reduction in travel and logistics expenses, while potentially increasing the number of such studies performed and generally improving the quality of more studies.
Telepathology within toxicologic pathology will in all likelihood increase in use and applications, as industry awareness grows of this new technology. It can bring with it the potential for better science and cost reductions in the development of new treatment modalities. Benefits of this new real-time telepathology technology may extend as well to pathology training programs that have traditionally used lectures and personal meetings requiring dual- and multiheaded microscopes.
Regulatory Issues of Telepathology in Toxicologic Pathology
Since telepathology in preclinical studies is similar in nature to study notes, there is no immediate Good Laboratory Practice (GLP) component. However, as telepathology is inherently translatable to recorded images, we believe it may become a desirable component of regulatory submissions in the future. Indeed, informal shared experience from colleagues involved in drug development suggests that historically the Food and Drug Administration (FDA) has accepted the submission of imaging data, when included as non-GLP components within a GLP study (similar to immunohistochemistry end points, electron microscopy, or other special assessments using nonvalidated assays), so long as the imaging data acquisition was demonstrated to be scientifically rigorous with appropriate controls. On this note, improvements in technology operation, ensuring image quality across all remote viewer locations, and calibration of quantitative toolsets will continue to be necessary as new applications are identified. By being integrated within an existing clinical microscope and utilizing traditional microscope components that do not alter the optical view of the tissue, the new telepathology system may facilitate the pathology workflow by enabling discussions and collaboration among remote parties without entailing lengthy regulatory and validation requirements beyond the existing GLP guidelines for laboratory equipment.
Conclusions
In conclusion, the availability of relatively inexpensive microscope-integrated telepathology systems now permits a much more feasible, cost-effective, and practical use of telepathology in biomedical research and preclinical studies as an alternative to travel of pathology experts or shipping slides. It can facilitate more informed decision-making relating to study results and strategic business steps in a shorter period of time. In addition, this technology facilitates good science by conveniently supporting informal and formal consultation and pathology peer-review consistent with best practices of toxicologic pathology. We believe that data will accumulate from peer-review sessions, pathology working groups, and other applications that will enrich the science that can be supported by this technology. With the growing use of telepathology in preclinical studies, regulatory agencies will accept and provide guidance on use of this and other telepathology modalities.
Authors’ Contribution
Authors contributed to conception or design (GS, AN, DR, RM, MR); data acquisition, analysis, or interpretation (GS, AN, DR, RM, MR); drafting the manuscript (GS, AN); and critically revising the manuscript (GS, AN, DR, RM, MR). All authors gave final approval and agreed to be accountable for all aspects of work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.S. and D.R. are the employees of Augmentiqs. A.N. serves as a consultant for Augmentiqs. R.M. serves as an advisor and champion of new technologies that have toxicologic pathology applications.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
Crissman, J. W., Goodman, D. G., Hildebrandt, P. K., Maronpot, R. R., Prater, D. A., Riley, J. H., Seaman, W. J., and Thake, D. C. (2004).
Best practices guideline: Toxicologic histopathology.
Toxicol Pathol 32, 126–31.
Hanna, M. G., Pantanowitz, L., and Evans, A. J. (2015).
Overview of contemporary guidelines in digital pathology: What is available in 2015 and what still needs to be addressed?
J Clin Pathol 68, 499–505.
Morton, D., Sellers, R. S., Barale-Thomas, E., Bolon, B., George, C., Hardisty, J. F., Irizarry, A., et al. (2010).
Recommendations for pathology peer review.
Toxicol Pathol 38, 1118–27.