Toxicologic pathology is the art of assessment of potential adverse effects at the tissue level in pre-clinical studies. In the case of biomaterials and medical devices, the toxicologic pathologists assess the safety (biocompatibility) and efficacy (conditions of the use) of the implantable materials. Proper assessment of biocompatibility of biomaterials is of utmost importance, since it helps to determine their safety after implantation in humans. Biomaterial-related toxicity can be attributed to several factors, including for example leachable compounds from the material leading to thrombosis or carcinogenesis, or biodegradation of the material causing changes in its physical and compatibility properties. Evaluation of biocompatibility and biofunctionality involves assessment of cytotoxicity, allergic responses, irritation, inflammation and systemic and chronic toxicity. In many of these assessments, the toxicologic pathologist has an important role in determining product safety and potential toxicity. In this article, we review the special needs for proper toxicologic pathology assessment of biomaterials and degradable polymers. We review common adverse effects expected with biomaterials and describe their pathological picture and their clinical relevance. We also introduce a novel compact MR imaging technology as a tool for assessing biocompatibility and efficacy of implanted biodegradable materials, since it allows for the longitudinal imaging and quantification of inflammation in vivo caused by the device implantation, and enabling general inspection of shape, location and integrity of the device in vivo. Since the MR imaging technique is non-invasive, the effects of the implantable device can be monitored longitudinally in the same animal without perturbation of the pathology.
Keywords:
toxicologic pathology; histopathology; MRI; safety assessment
Introduction
Toxicologic pathology involves evaluation of biological tissues for adverse effects, and is especially important in pre-clinical studies. When biomaterials and medical devices are implanted in tissues, the toxicologic pathologists are responsible for evaluating the safety (biocompatibility) and efficacy (conditions of the use) of the implantable materials. The term “biocompatibility” encompasses a broad spectrum of biological notions. In addition to referring to the fact that the biomaterial should lack cytotoxicity, it also relates to its biofunctionality, or its ability to support cell–biomaterial interactions in the tissue milieu where the biomaterial is applied.
The perfect biomaterial preferentially should be prepared from a polymer which has the following characteristics[2]:
• Does not cause an inflammatory reaction
• Is metabolized without leaving traces after accomplishing its goal
• Processed with ease to its final form
• Has long shelf life
• Sterilized easily
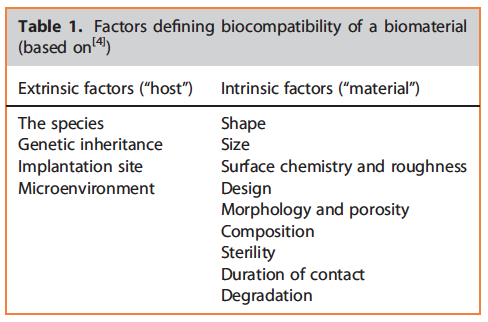
Appropriate inspection of biocompatibility of biomaterials is of utmost significance, since it allows to estimate their safety and function after implantation in humans.[3] This is especially true for biomaterials, considering the fact that they are implanted in humans for long periods and reside in close contact with biological tissues.[4] Biocompatibility of biomaterials depends on various parameters, which can be divided into internal (the material itself) and external (the host) parameters.[4] These factors which define biocompatibility are summarized in Table 1.[4]
Many factors can lead to biomaterial-related toxicity, which include for example thrombosis or carcinogenesis induced by leachable compounds from the material, or biodegradation of the material which alters its physical and compatibility characteristics.[5] Additional factors such as immunologic reactions of the host or accidental introduction of biofilms also play a major role in toxicity of biomaterials.[6]
Unfortunately, toxicity evaluation of biomaterials is a complex process, which involves both in vitro and in vivo methods. In vitro cell culture tests, usually involving permanent cell lines, are often applied to screen for the biocompatibility of a compound. The International Organization of Standardization (ISO) 10993 provides a series of standards for assessing biocompatibility of medical devices.[7] These standards are presented in 20 different parts, which can be accessed in the following web address: http://www.iso.org/iso/home.
The process of biocompatibility and biofunctionality evaluation usually involves assessment of cytotoxicity, allergic responses, irritation, inflammation and systemic and chronic toxicity.[5] In many of these assessments, the toxicologic pathologist has an important role.
Toxicologic Evaluation Of Biomaterials
An important difference between the toxicologic pathology evaluation of drugs and biomaterials is that biomaterials always cause tissue reaction, due to their close interaction with the tissue for a long time. It is also for this reason that the response to biomaterials is often time dependent and not dose dependent. Other factors that should be taken into consideration are the implantation procedure, which often involves tissue trauma and the placement of sutures. Additionally, secondary infection can result from the operative procedure or from contamination of the implanted device.[6]
The toxicologic pathologist is often asked to evaluate implantation tests, which are intended for determining the local effect of devices on tissues or body fluids.[6] These tests are routinely directed at paravertebral or hind-limb muscle of rabbits or less frequently rats.[6] These tests often pose a special challenge to the toxicologic pathologist due to unique confounders and problems, which are summarized in Table 2. Species-related differences in response to implanted material should also be taken into account, and it is our recommendation that in comparable circumstances when relative severe host reaction is seen in one species (e.g. pig), then another animal species should be tested for safety, in order to exclude potential particular sensitivity in one species. In addition, until this aspect is clarified, it is recommended that no clinical testing with that compound will be performed.
The histopathology evaluation form for implanted materials can be found in the paper by Schuh,[6] which represents a modification of the historical evaluation form. When evaluating pathology of biomaterials, special emphasis should be given for providing a detailed summary of pathology results in addition to semi-quantitative scores, since many parameters are not included in this scoring system, including for example hemorrhage, edema and the different cell populations in the infiltrate.[6] Additionally, it is obligatory that the pathologist will also add to the semi-quantitative scoring a detailed interpretative assessment, in order to have a clear understanding of the characteristic and potential significance of the findings. Detailed and annotated photographic documentation should be encouraged, especially when dealing with unusual findings.
Upon histopathologic evaluation, it is important that the pathologist will adopt the terminology currently recommend by the International Harmonization of Nomenclature and Diagnostic (INHAND) criteria for lesions.[8] Also, according to the “Best Practices for Reporting Pathology Interpretations within GLP Toxicology Studies,” published by the Society of Toxicologic Pathology (STP), when evaluating the histological slides from a study, the study pathologist should have access to the study protocol and all protocol amendments, all study data including the intended pharmacologic target and mechanism of action, in-life study data, clinical pathology data, organ weight data, necropsy findings, toxicokinetic information and (when possible) data from previous studies with the same test article.[9]
The importance of host immune response to the implanted material has gained much attention in recent years. Therefore, immunotoxicology of biomaterials should be part of the histopathological evaluation. To address this need, evaluation of hematopoietic and lymphoid tissues should be performed.[6]
When designing the toxicology protocol, special issues that should be taken into consideration include scheduling interim sacrifices, using concurrent untreated samples, and histopathological examination of organs that may suggest potential systemic toxicity, such as liver, kidneys, spleen, lungs, regional lymph nodes and heart.[3,10] Scheduled interim sacrifices will allow the toxicologic pathologist to determine whether there is time-related decrease in the inflammatory reaction. When assessing the longterm biocompatibility, an example of interim sacrifice schedule may include sacrifices at 1, 3, 6 and 9 months.[11]
Since the implantation location may not have a uniform structure, it is preferable to examine more than one tissue slice from the same tissue specimen (i.e. between 2 and 4 slices). When sampling tissues for biocompatibility assessments, it is important to consider that any removal of the implant material may remove all or the majority of the reactive tissue and distort the implant space. Therefore, according to the physical properties (i.e. soft versus hard) of the implant, a decision should be taken if need for expensive resin embedding is required.[6]
It has been shown previously that biomaterials can cause malignant neoplasms in animal models.[5] Such an effect can be due to a surface effect of the implanted material, or due to leaching of a chemical carcinogen.[5] Due to this risk, long-term carcinogenicity studies should be performed with laboratory animals.
The pathologist should pay attention to any potential presence of components in the adjacent tissue that may suggest adverse reactions, and are not mentioned in the regulatory required histopathology scoring for biocompatibility. Examples include the presence of mineral or pigment deposits that may further require use of histochemical stainings in order to elucidate the nature of the deposits; presence of thrombi which may suggest a test compound-related prothrombotic event[12]; foreign-body reaction which requires effort to identify the nature of the foreign material (i.e. whether derived from fragmentation of the implanted material or just from remnants from cotton gauze swabs used during the operation procedure); presence of lymphoid follicle aggregations which may suggest an immunemediated reaction[13]; presence of bacterial colonies which may derive from test compound not prepared in sterile procedure, or from an adjacent bacterial leaking organ (i.e. intestine).
The potential challenges of evaluating biocompatibility of biomaterials are exemplified by a recently reported routine transcoronary preclinical safety assessment trial in pigs. Pathology evaluation revealed the occurrence of incidental myocardial intravascular foreign-body granulomas.[14] The microsopic appearance together with Ziehl Neelsen and pancytokeratin stains and polarized light microscopy revealed that these granulomas were caused by cotton fibers, as may be seen in cotton gauze swabs and cotton sutures. This observation emphasizes that foreign-body embolization can occur during invasive vascular interventions, which may lead to foreign-body reaction and misinterpretation of drug safety results.
Biodegradable polymers are attractive candidates for medical applications, owing to their transient nature, thus eliminating the need for surgical removal.[5] For this reason, they have been developed for use in a large number of applications, including sutures, drug delivery systems, stents and more.[5] They are especially important for orthopaedic applications by allowing polymers to slowly degrade and transfer load to healing bones.[15] Biodegradable polymers are characterized by degradation into normal metabolites or into products which can be completely eliminated from the body.[5] Biodegradable polymers degrade and resorb in two phases: the first phase consists of hydrolization of long polymer chains to shorter ones. This first phase allows reduction in molecular weight, without affecting the physical properties of the device, since the crystalline regions hold the device matrix together. As water starts fragmenting the device itself, physical properties are affected.[2] The second phase consists of the normal reaction of the relevant tissue to metabolize and phagocytize the short polymer chains, which leads to rapid loss of polymer mass.[2,16] Although biodegradable polymers avoid the long-term foreign-body reaction that is seen with other long-standing implanted devices, there are also adverse effects associated with the degradation processes. These adverse tissue reactions may result from crystalline remnants or a decrease of pH, which depend on the molecular structure of the biomaterial.[17,18]
Special attention should be paid to the layer of macrophages, located at the interface between the tissue and the implant. If this layer is not detected, or there is lining of necrotic macrophages, it may suggest that the implanted material or one of its components are potentially toxic to the macrophages. This may cause delayed elimination of the test compound, or even permanence of the implanted test compound, even following relative long-term follow-up (i.e. several months after implantation).
Foreign-body Reaction
Implantation of a biomaterial is always accompanied by an in- flammatory process, which is aimed to prevent tissue damage, isolate and destroy the foreign material and to initiate the repair process.[4,19] This reaction can be divided into two phases: the acute phase response and the chronic phase response, each can be further divided into two separate stages. The acute phase response: This is an acute inflammatory process, lasting from several hours to days, which is characterized initially by changes in the microvasculature, leading to dilation of blood vessels and increased blood viscosity. Proteins leak from the injured blood vessel and together with tissue fluid proteins cover the foreign material, forming a strong and adhesive protein layer (Fig. 1A).[20–22] Concomitantly, leukocytes start to infiltrate the adjacent interstitial tissue. The cells which characterize this stage are mostly neutrophils, but monocytes and lymphocytes can also be observed (Fig. 1B).[4,23] The acute phase reaction is mostly mediated by interleukin (IL)-1, IL-6 and tumor necrosis factor-α, secreted by monocytes, macrophages, fibroblasts and endothelial cells.[4]
The chronic phase response: This phase is dominated by proliferation of fibroblasts and macrophages.[4] Macrophages may fuse to form multinucleated foreign-body giant cells, which accumulate around the biomaterial (Fig. 1C). In parallel, fibroblasts produce collagen which forms a fibrous capsule around the implant (Fig. 1D).[24] This capsule, although not-necessarily indicating a non-biocompatibility state, may harm the diffusion of an encapsulated drug within the tissue and reduce supply of oxygen and nutrients to encapsulated cells.[20]
Granuloma formation: If the implant remains as a foreign body in the tissue for a long time, it may result in granuloma formation.[4] This process is characterized by accumulation of activated macrophages and lymphocytes, abundance of multinucleated giant cells and a clinical phenotype of edema and pain. In case the biomaterial is toxic to macrophages, they die and release enzymes to the surrounding tissue, causing tissue damage that may culminate in necrotic tissue.[21,25] When evaluating the histopathology of foreign-body reactions, several grading methods can be used. One such grading system is the one suggested by Duranti et al., which follows the stages of granuloma formation as described above[26]:
• Grade I: slight reaction with a few inflammatory cells
• Grade II: clear inflammatory reaction with one or two giant cells
• Grade III: fibrous tissue with inflammatory cells, lymphocytes and giant cells
• Grade IV: granuloma with encapsulated implants and clear foreign-body reaction
An extraordinary case of a relatively common chronic inflammatory reaction is the process that ensues after injection of chemically modified hyaluronic acid fillers.[22] Since hyaluronic acid is identical in all species, the reason for this foreign-body-like reaction is still obscure. Some researchers have attributed this reaction to contamination with residual bacteria or avian proteins.[27] Others have suggested that this reaction is due to the chemical crosslinking process, which leads to changes in the three-dimensional structure of the injected compound.[27] It is therefore evident that not all foreign-body reactions for injected biomaterials are directly related to the biomaterial itself, which can be inert, but to modifications prior to injection or due to contamination in the production or injection process.
Implications Of The Adverse Effects Caused By Implantable Biomaterials
Fibrosis encapsulation
Subcutaneous glucose sensors have been developed for routine glucose monitoring in diabetics and intend to replace the inconvenient external monitoring by skin pricking.[1,28] However, implantation of such devices leads to foreign-body reaction and consequently to fibrous encapsulation. This capsule prevents the transport of low molecular weight analytes, such as glucose, to the sensor.[1,29] Several solutions have been developed, including the employment of different coatings to the sensor, enhancement of neovascularization at the implant site or the application of anti-inflammatory agents.[1]
Stent restenosis
Coronary stent implantation is commonly used to treat ischemic heart disease, but stent restenosis is a substantial problem.[1] The stent protrudes into the endovascular tissue and can lead to an inflammatory reaction causing neointimal proliferation and restenosis.[12,30,31] To prevent this process, drug-eluting stents have been developed, including immunosuppressive drugs, antiproliferative drugs and antimigratory drugs.[1] Sirolimus-eluting stents are currently the most commonly used stents.[32]
Calcification
Calcification of implantable devices can cause major functional impairments. The calcification process is especially problematic in the case of bioprosthetic heat valves, limiting their lifetime to approximately 15 years.[1] This process is probably initialized by tissue cell devitalization, leading to the formation of crystals from membrane-derived phosphorus and calcium from the extracellular fluid.[33] In order to overcome this problem, several techniques have been developed, including the combination of implantable device and a drug delivery system or the modification of the preparation procedure and of the constituents of the implantable device.[1]
Injectable soft tissue fillers
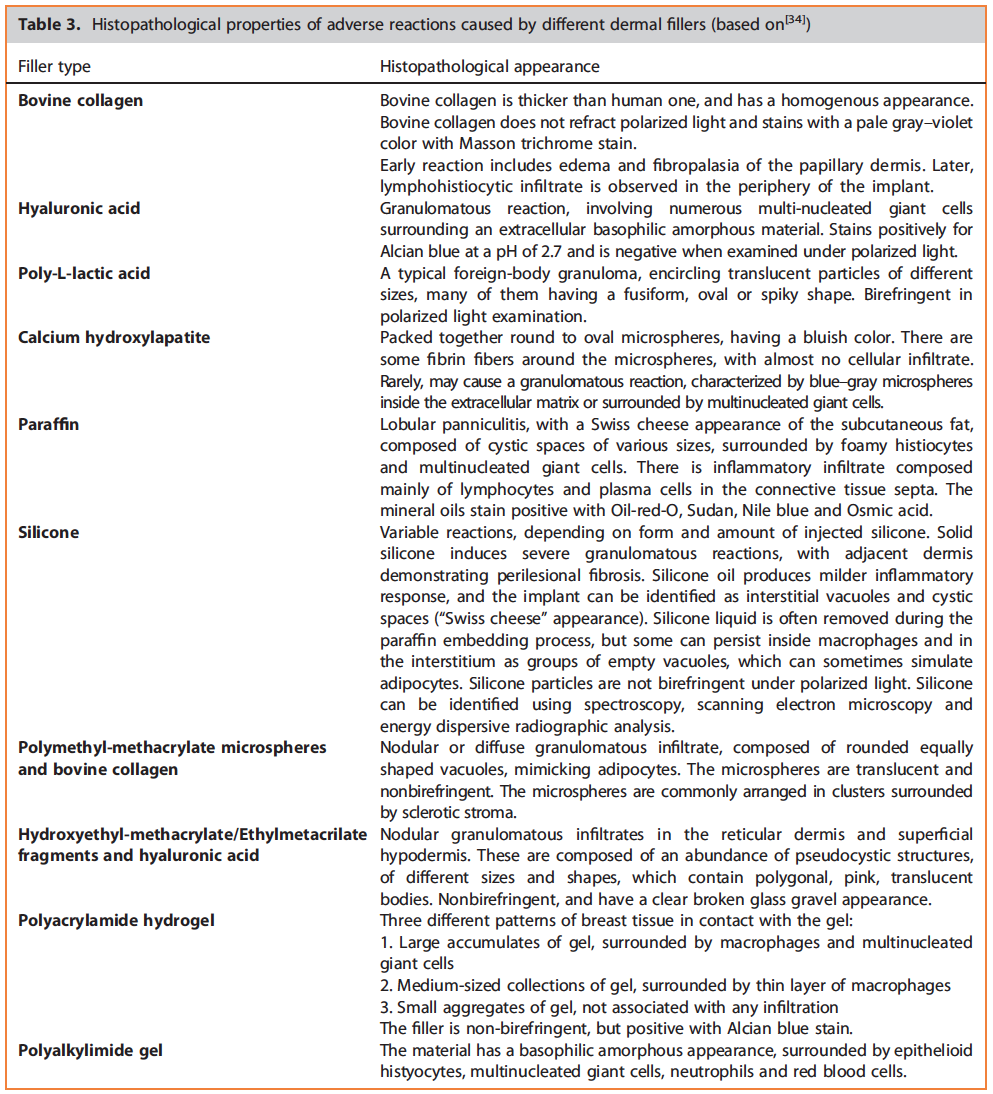
The use of filler agents for rejuvenation has been growing rapidly in recent years. Although there is a large variety of fillers, all of them can cause adverse reactions. In general, fillers can be divided into resorbable (within months or years) and permanent. While the adverse effects caused by resorbable fillers often resolve spontaneously, the ones caused by permanent fillers often show no spontaneous resolution.[34] In the case of adverse reactions from fillers, histopathology evaluation has a special role, since it can identify the specific filler which was used, and by that determine the inflammatory reaction, helping to guide the appropriate treatment.[34] This is especially important in litigation cases, when identifying the specific filler that caused the adverse effect is of crucial significance. Table 3 describes the different pathological reactions caused by each filler, demonstrating the large variety of pathological characteristics that can be induced by biomaterials, and emphasizes the importance of appropriate pathological evaluation. This evaluation should often involve special stainings such as Masson trichrome stain, Alcian blue, Oil-red-O and the use of polarized microscopy, spectroscopy, electron microscopy or energy dispersive radiographic analysis.
Application of In Vivo And Ex Vivo Compact Mri in Preclinical Safety and Efficacy Studies
It is now possible to perform isotropic 3D in vivo magnetic resonance imaging (MRI) images of rodents as well as images of formalin-fixed whole tissue specimens from rodents and larger animals with compact, high-resolution MRI scanners that can be safely utilized in an animal room, a histology laboratory or even a pathologist’s office. Unlike conventional superconducting MRI systems, these compact MRI instruments are self-shielded, meaning they have no external magnetic fringe field and do not require the infrastructure, such as shielded rooms, cryogens and extensive electrical and plumbing infrastructure to support the magnetic field. Instead, these compact systems simply plug into standard electricity outlets, are self-shielded and can be operated by technicians without any prior imaging expertise. With this technique, non-invasive, repetitive evaluation of tissue changes over time in experimental animal models is possible. Imaging of whole fixed tissue samples allows a detailed examination of multiple digital slices with subsequent volumetric measurement of three-dimensional structures while leaving the specimen intact for subsequent conventional H&E histology. Compact MR-based technology can also be utilized in biocompatibility and efficacy studies with implanted biodegradable materials.
In vivo MRI was performed using the M2™ , a compact, highperformance MRI system (Aspect Imaging, Israel), equipped with a 60 mm rat body coil. Rats were kept anesthetized with 2% isoflorane in O2 on a specially designed heated bed where physiological signals were monitored throughout the experiment to ensure the animals’ well-being. High-resolution ex vivo MRI was performed using a 20 mm RF coil on the same compact, high performance MRI system. In this configuration, the system allows for high-throughput, automated scanning of up to 10 fixed samples without technician intervention. Briefly, fixed samples are placed into specially designed disposable capsules which are given a unique barcode. The operator assigns pre-defined MR protocols from a list to the barcoded sample, according to the organ or pathology being investigated. Up to 10 capsules, each with their own application-specific imaging protocol, can then be loaded into the system, which automatically advances the sample into the MRI unit, executes the specified imaging protocol and then ejects the capsule. The next sample is then automatically advanced, its unique imaging protocol is executed and the process continues automatically. No technician intervention is required throughout the process so samples can be left unattended to be scanned overnight. For detailed MR acquisition parameters, see figure legends. Segmentation and quantification of the 3D data sets were done using Vivoquant (In Vicro, Boston, USA).
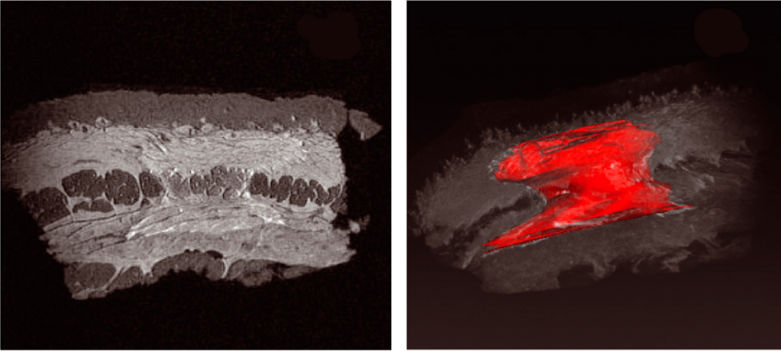
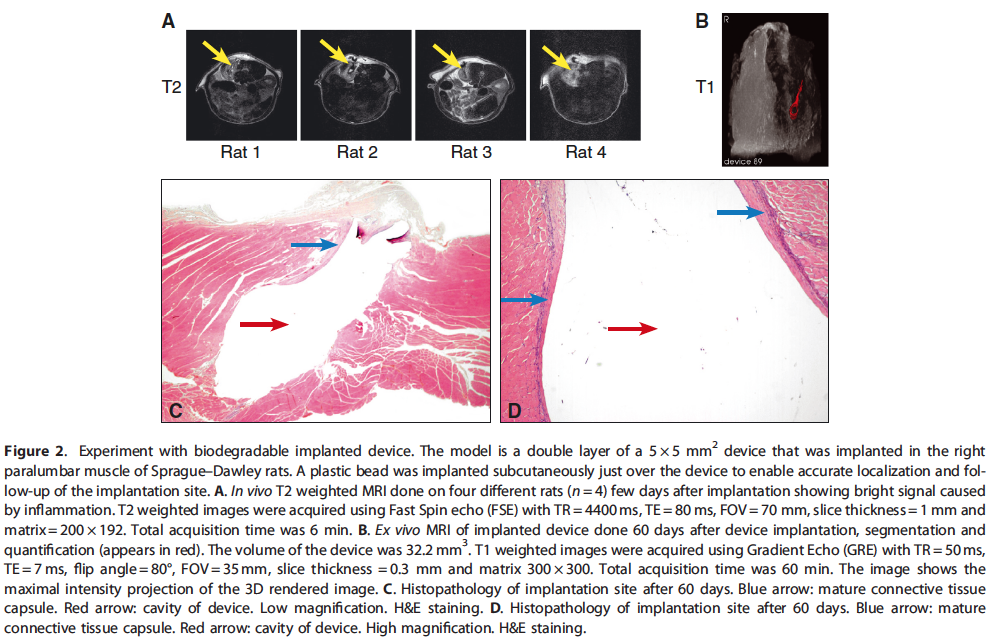
Two experimental models in which the M2 system was used to investigate the tissue reaction to biodegradable compounds are presented. In the first model, a device implanted in the paralumbar muscle of rats was evaluated in vivo by compact MRI, which detected inflammation related to the implanted device. Compact MRI was further used to follow the progressive reduction in the in- flammatory process. The in vivo MRI was able to demonstrate the presence of the test device in the para-lumbar muscles up to 16 weeks post implantation. Using ex vivo MRI, it was possible to quantify accurately the device volume and shape. Combination of in vivo and ex vivo MRI with histopathology can therefore be used to shed light on reaction to implantation and bio-degradation of devices. For details of the obtained results in our experimental model, see Fig. 2.
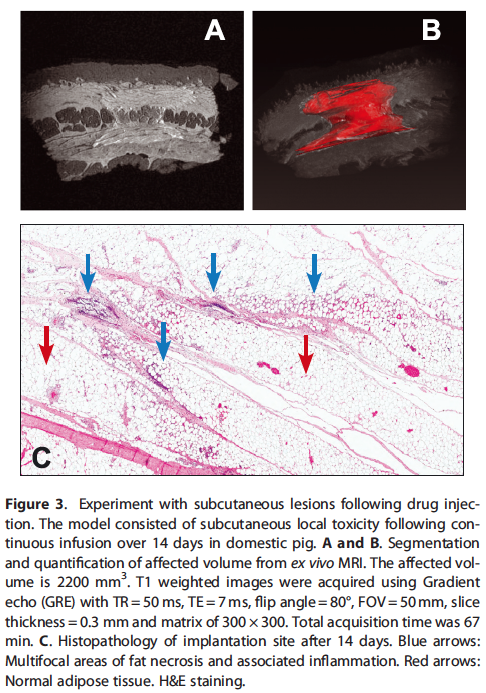
In the second model, the M2 system was used to evaluate the extent of subcutaneous lesions following drug injection. The model involved the investigation of subcutaneous local toxicity following continuous infusion over 14 days in domestic pig. The ex vivo MRI was effective in identifying the location and quantifying the extent of subcutaneous necrosis and inflammation caused by the drug. Applying this method on multiple tissues samples derived from different dose formulations provided a quantitative determination of relative irritancy of different injected formulations. For details of the obtained results in our experimental model, see Fig. 3.
It was proved that the new compact MRI evaluation technique is especially beneficial for biodegradable materials which necessitate long-term non-invasive monitoring of the experimental animals, and is a complementary adjunct to toxicologic pathology. This imaging technique has unique advantages by allowing the performance of longitudinal studies with reduced number of animals, in contrast to conventional studies which require interim necropsies, as well as being effective in identifying the location and quantifying the extent of tissue damage caused by the implanted material through the generation of high-resolution three-dimensional digital data sets.
Conclusions
The use of biomaterials is gradually increasing in clinical practice, and therefore, the toxicologic pathologist is expected to encounter an increasing number of preclinical safety studies with such devices. Therefore, proper knowledge on the special considerations that should be applied when evaluating biomaterials and degradable polymers should be acquired by the pathologist. Often, such evaluation necessitates the use of special stainings or other adjunctive techniques such as electron microscopy or spectroscopy. Recently, in vivo and ex vivo compact MRI evaluations are gaining popularity as adjuncts for classic histopathology assessments, and are especially tempting for use in biodegradabe materials, as they allow long-term quantitative assessments non-invasively without the need for interim sacrifices of animals.
References
[1] Y. Onuki, U. Bhardwaj, F. Papadimitrakopoulos, D. Burgess, J. Diabet. Sci. Tech. 2008, 2(6), 1003–15.
[2] J. Middleton, A. Tipton, Biomaterials 2000, 21(23), 2335–46.
[3] B. Vaisman, M. Motiei, A. Nyska, A. Domb, J. Biomedic. Mater. Res. Part A 2010, 92(2), 419–31.
[4] E. Fournier, C. Passirani, C. N. Montero-Menei, J. P. Benoit, Biomaterials 2003, 24(19), 3311–31.
[5] D. S. Katti, S. Lakshmi, R. Langer, C. T. Laurencin, Adv. Drug Deliv. Rev. 2002, 54(7), 933–61.
[6] J. Schuh, Toxicol. Pathol. 2008, 36(1), 63–9.
[7] ISO document, 10993, Biological compatibility of medical devices. http://www.iso.org/iso/home, 2012.
[8] P. C. Mann, J. Vahle, C. M. Keenan, J. F. Baker, A. E. Bradley, D. G. Goodman, T. Harada, R. Herbert, W. Kaufmann, R. Kellner, T. Nolte, S. Rittinghausen, T. Tanaka, Toxicol. Pathol. 2012, 40(4 Suppl), 7S–13S.
[9] D. Morton, R. K. Kemp, S. Francke-Carroll, K. Jensen, J. McCartney, T. M. Monticello, R. Perry, O. Pulido, N. Roome, K. Schafer, R. Sellers, P. W. Snyder, Toxicol. Pathol. 2006, 34(6), 806–9.
[10] W. Khan, S. Farah, A. Nyska, A. Domb, J. Control. Release 2013, 168(1), 70–6.
[11] G. Lemperle, V. Morhenn, U. Charrier, Aesthetic Plast. Surg. 2003, 27(5), 354–66; discussion 67.
[12] Y. Ramot, A. Nyska, G. Spectre, Drug Saf. 2013, 36(8), 585–603.
[13] C. Gopinath, Inflamm. Res. 1996, 45(Suppl 2), S74–8.
[14] Y. Ramot, G. Amir, E .P. Willenz, A. Nyska, Toxicol. Pathol. 2008, 36(3), 385–7.
[15] K. A. Athanasiou, C. M. Agrawal, F. A. Barber, S. S. Burkhart, Arthroscopy 1998, 14(7), 726–37.
[16] G. J. Buijs, B. Stegenga, R. R. M. Bos, J. Dent. Res. 2006. 85.
[17] M. S. Taylor, A. U. Daniels, K. P. Andriano, J. Heller, J. Appl. Biomater. 1994, 5(2), 151–7.
[18] M. Vert, S. Li, H. Garreau, Clin. Mater. 1992, 10(1–2), 3–8.
[19] H. Baumann, J. Gauldie, Immunol. Today 1994, 15(2), 74–80.
[20] B. Rihova, Adv. Drug Deliv. Rev. 2000, 42(1–2), 65–80.
[21] L. Tang, J. W. Eaton, Am. J. Clin. Pathol. 1995, 103(4), 466–71.
[22] P. Edwards, J. Fantasia, Clin Intervens Aging 2007, 2(4), 509–19.
[23] B. D. Ratner, J. Control. Release 2002, 78(1–3), 211–8.
[24] A. G. Mikos, L. V. McIntire, J. M. Anderson, J. E. Babensee, Adv. Drug Deliv. Rev. 1998, 33(1–2), 111–39.
[25] S. J. Williams, G. C. Farrell, Br. J. Clin. Pharmacol. 1986, 22(5), 610–2.
[26] F. Duranti, G. Salti, B. Bovani, M. Calandra, M. Rosati, Dermatol. Surg. 1998, 24(12), 1317–25.
[27] P. Andre, J. Eur. Acad. Dermatol. Venereol. 2004, 18(4), 422–5.
[28] H. E. Koschwanez, W. M. Reichert, Biomaterials 2007, 28(25), 3687–703.
[29] N. Wisniewski, B. Klitzman, B. Miller, W. M. Reichert, J. Biomed. Mater. Res. 2001, 57(4), 513–21.
[30] A. Gaspardone, F. Versaci, Am. J. Cardiol. 2005, 96(12A), 65L–70L.
[31] Y. Ramot, A. Nyska, Toxicol. Pathol. 2007, 35(2), 208–25.
[32] G. W. Stone, J. W. Moses, S. G. Ellis, J. Schofer, K. D. Dawkins, M. C. Morice, A. Colombo, E. Schampaert, E. Grube, A. J. Kirtane, D. E. Cutlip, M. Fahy, S. J. Pocock, R. Mehran, M. B. Leon, N. Engl. J. Med. 2007, 356(10), 998–1008.
[33] F. J. Schoen, R. J. Levy, Ann. Thorac. Surg. 2005, 79(3), 1072–80.
[34] L. Requena, C. Requena, L. Christensen, U. Zimmermann, H. Kutzner, L. Cerroni, J. Am. Acad. Dermatol. 2011, 64(1), 1.