Background: Alpha-glycosyl isoquercitrin (AGIQ) is widely used as an anti-oxidative food additive in many products. Nevertheless, information on its safety and toxicity is still very limited. A 90-day toxicity study in rats and comprehensive genotoxic studies have proven AGIQ to be safe.
Aim: The goal of this study was to assess the safety of use of AGIQ in infant formula. To test for potential adverse effects on growth or other safety issues specific for young animals, we performed a 10-day and a 4-week study in preweaning Göttingen minipigs.
Method: Newborn minipigs were treated four times per day with oral AGIQ (0, 100, 300, or 1000 mg/kg/day) for 10 days or 4 weeks.
Results: All animals remained in good health throughout both studies, and there were no treatment-related signs of toxicity or abnormal findings in blood parameters. In the 4-week study, yellow coloration of the bones was seen in all animals in the high-dose group with no related histological findings. Hepatocellular and sinusoidal/Kupffer cell iron deposition was seen in the liver of all animals in both studies, as expected after the routine administration of supplemental iron to newborn animals. There was sufficient evidence of systemic exposure based on plasma levels of AGIQ metabolites.
Conclusion: Taken together, these findings show that oral administration of AGIQ in reconstituted milk supplement to preweaning Göttingen minipigs for 4 weeks at up to 1000 mg/kg/day does not result in any adverse treatment-related effects and further support the safety of AGIQ as a food additive.
Introduction
Quercetin is a flavonol that is abundant in plants, and together with its glycosides has proven to exert a large number of positive biological functions, such as anti-inflammatory and anti-oxidative effects,1,2 in addition to hepatoprotective, anti-cancer properties and improvement of metabolic disturbances.3–6 Isoquercitrin is a glycoside of quercitrin with a higher bioavailability than quercetin; however, natural amounts of isoquercitrin in plants are low. Large quantities of isoquercitrin can be produced via enzymatic hydrolysis of rutin followed by transglycosylation with dextrin and cyclodextrin glucanotransferase.7–9 The product of this enzymatic process is termed enzymatically modified isoquercitrin or alpha-glycosyl isoquercitrin (AGIQ). AGIQ has been found to possess a higher bioavailability than quercetin or isoquercitrin in humans10 and has proven pharmacological benefits in vivo, including tumor suppressive and anti-oxidative effects.11–19
AGIQ has been used as a natural food additive in Japan since 1987 and is in wide use as an anti-oxidant in a large number of products, ranging from beverages, frozen dairy products and puddings to chewing gums and canned soups.20 Following the Japanese approval in 1996, the Expert Panel of the Flavor and Extract Manufacturers Association granted AGIQ a generally recognized as safe status in 2005, and the same status was granted by the US Food and Drug Administration (FDA) in 2007.21 However, although it is in wide use and sold as a food additive, information on its safety and toxicity is still limited, and based mostly on studies that used AGIQ of low purity or on studies that were not good laboratory practice (GLP)-compliant.21–24 In anticipation of the potential use of AGIQ in infant formula, we conducted this study in preweaning animals to test for potential adverse effects on growth or other safety issues specific for young animals.
According to Constable et al.,25 who recently extensively discussed an integrated approach to the safety assessment of food additives in early life, currently there is neither scientific consensus nor guidance from regulatory bodies on the most suitable animal species to address toxicological data gaps in the early life period. It is suggested that the early life stage of pigs is close to human early life stage.26 Since there is only limited safety information on isoquercitrin and AGIQ, we have performed a comprehensive evaluation process of highly purified AGIQ in a series of GLP-compliant tests in accordance with US FDA, European Food Safety Authority, and the Organisation for Economic Co-operation and Development (OECD) guidelines. As part of this evaluation process, we have performed a 90-day repeat dose toxicity and single dose toxicokinetic (TK) study in Sprague-Dawley rats, which showed no signs of toxicity.27 A genotoxicity assessment revealed that both AGIQ and isoquercitrin caused mutations in several bacterial strains, but no mutagenic potential was evident in any of the several tissues when tested in transgenic mice. Furthermore, although isoquercitrin tested positive in the chromosomal aberration test in Chinese hamster ovary (CHO) cells, all additional in vivo and in vitro assays did not show any numerical or structural chromosomal or DNA damage.28 Taking into consideration the above-mentioned rationale for the suitability of the minipig model, here we report the results of safety and TK studies performed in preweaning Göttingen minipigs. A preliminary 10-day study was performed to aid the selection of the doses for the main 4-week toxicity study.
Materials and methods
Animal husbandry and maintenance
Pregnant Göttingen minipigs were obtained from Ellegaard Göttingen Minipigs, Denmark, and were allowed an acclimatization period of at least 4 weeks prior to the expected farrowing date. The test animals were unweaned piglets selected from the litters produced. The piglets were initially housed in litters (with sow) for at least the first 24 h. Thereafter, they were housed with their litter mates (without sow). The first study treatment was given at the age of 2 days. Uniferon® iron III dextran (Pharmacosmos, Denmark) was administered on postnatal day (PND) 1 or 2 (0.5 mL/kg administered intramuscularly into a hind leg).
The animals were housed in indoor pens with farrowing rails, separate piglet creep areas, and infrared heat lamps as appropriate. Temperature was monitored and maintained within the range of 15–24°C, and artificial lighting dictated a 12-h light/12-h dark cycle. For the first approximately 24 h after birth (PND 1/2), the piglets were allowed to suckle from the sow. Afterward, the piglets were given reconstituted milk supplement (Volac Faramate, Volac International Ltd, Devon, UK) mixed at a rate of 150 g/L of mixed milk every 3 h daily. The animals were given free access to potable water from the public supply. The study was conducted in Envigo CRS Limited, Woolley Road, Alconbury, Huntingdon, Cambridgeshire, UK, in accordance with the applicable sections of the United Kingdom Animals (Scientific Procedures) Act 1986, Amendment Regulations 2012 (the Act).
Experimental design
As a preliminary assessment of systemic toxicity and TK, a 10-day study consisted of three treated groups without a control group with five animals in each group (Table 1). Daily dosing was from PND 3 to PND 12. A subsequent 4-week study consisted of three treatment and one control groups with 7 animals in each group including 4 (2 male + 2 female) main study animals and 3 animals for TK (Table 1). Daily dosing was from PND 3 to PND 30. AGIQ (consisting of >97% AGIQ and 0.13% quercetin) was supplied by San-Ei Gen F.F.I, Inc. (Osaka, Japan) and was provided orally four times daily (every other feed) with a volume dose of 5 mL/kg body weight. Initially, the first dose was administered via oral gavage in a small amount of Volac Faramate reconstituted milk supplement to facilitate TK sampling. Thereafter, the dose was given in a small amount of reconstituted milk supplement via syringe before offering untreated milk via bottle-feeding. Any dose not taken voluntarily via syringe was administered by oral gavage at the end of the dosing session.
Viability, clinical signs, weight, and food consumption
Animals and their pens were inspected visually at least twice daily for evidence of reaction to treatment or ill-health at least twice in the approximately 24 h after birth, prior to weaning from the sow, and at least eight times daily (at times of feeding) thereafter, and detailed observations were recorded daily during and after each occasion of dosing. The piglets were weighed individually within 24 h after birth, daily from PND 3 and before necropsy in the 10-day study, and daily from PND 3 to 9, twice weekly from PND 9 and before necropsy in the 4-week study. In the 10-day study, the weight of food (milk) supplied to each animal and that remaining was recorded daily from PND 3.
Hematology, biochemistry, coagulation, and urinalysis
Hematology, blood chemistry, coagulation, and urinalysis tests for the 10-day and 4-week studies are listed in Supplemental Table 1. Blood for hematology, biochemistry, and coagulation was collected via the jugular vein pretreatment and at termination in the 10-day study and on day 14 and day 28 in the 4-week study. Urine samples were collected from all animals in the 4-week study before termination.
Necropsy and tissue handling
Piglets were sedated using isoflurane followed by overdose of sodium pentobarbitone solution (200 mg/mL) by intravenous injection followed by exsanguination. All animals were subject to a detailed necropsy, and all organs and tissues were examined for grossly visible lesions. Organs weighed, tissues fixed, and a comprehensive list of tissues examined microscopically are listed in Supplemental Table 2. Tissues were preserved in 10% neutral buffered formalin, except eyes and testes, which were fixed in Davidson’s fluid and modified Davidson’s fluid, respectively. Bone marrow smears were air-dried and subsequently fixed in methanol.
For histology, tissue samples were dehydrated, embedded in paraffin wax, and sectioned at a nominal four- to five-micron thickness. For bilateral organs, sections of both organs were prepared. A single section was prepared from each of the remaining tissues required. Sections were stained with hematoxylin and eosin (H&E). Findings were either reported as “present” or assigned a severity grade.29 A single section was prepared from the livers of representative animals and stained with Prussian Blue for the presence of iron deposits.
Toxicokinetics study
In the preliminary10-day study, plasma samples for TK were obtained from all treated animals after the first of four daily doses on PND 3 (the first day of treatment) and after the first dosing on PND 12 (day 10 of dosing). For the main 4-week study, plasma samples were obtained from only one or two minipigs per sex after the first of four daily doses on PND 3 and at study termination on PND 30. The time of sampling after dosing was 0.5 h, 2 h, 6 h (prior to dosing), and 24 h for both studies; all blood samples were collected via the jugular vein. Plasma samples were analyzed for quercetin, isoquercitrin, and quercetin-3-glucuronide by a quantified liquid chromatographic-tandem mass spectrometric (LC-MS/MS, API 4000, Sciex) method with a limit of quantitation of <20.0 ng/mL. All summary data reported in tables are based on rounded numbers. Due to the paucity of quantifiable data, statistical analysis was not considered appropriate. Standard deviations were calculated based on a minimum of three data points.
Results
Survival, clinical observations, hematology and urinalysis parameters, and body and organ weight
Ten-day study
All animals remained in good health throughout the study, and there were no treatment-related signs of toxicity. Body weight gains were similar in all groups, and there was no apparent effect of treatment. Food consumption (milk) was variable between animals with no apparent treatment-related effects. There was some degree of inter-animal variability in organ weights; however, there were no notable differences between the groups and individual organ weights were within normal ranges for this age. There were no apparent effects of treatment on hematological parameters (data not shown).
Four-week study
All animals remained in good health throughout the study, and there were no treatment-related signs of toxicity. Body weight gain in almost all treated groups was slightly less than in controls over the duration of the study (Table 2). Mean daily body weights are provided in Supplemental Table 3.
There was some degree of inter-animal variability in platelet count levels due to the age of the animals that was considered nonadverse, and some degree of inter-animal variability in alkaline phosphatase levels that was attributed to the blood sampling procedures (data not shown). The urine in group 4 after 4 weeks of treatment was darker (medium yellow) in 4/4 animals when compared to controls (1/4), but there were no differences in urinalysis parameters between treated animals and controls that were attributable to treatment.
Macroscopic and microscopic observations
Ten-day study
The macroscopic examination performed after 10 days of treatment revealed no AGIQ-related lesions. Abnormal coloration of the lungs was seen in most animals and was due to the euthanasia procedure. The incidence and distribution of all findings were considered incidental and unrelated to AGIQ.
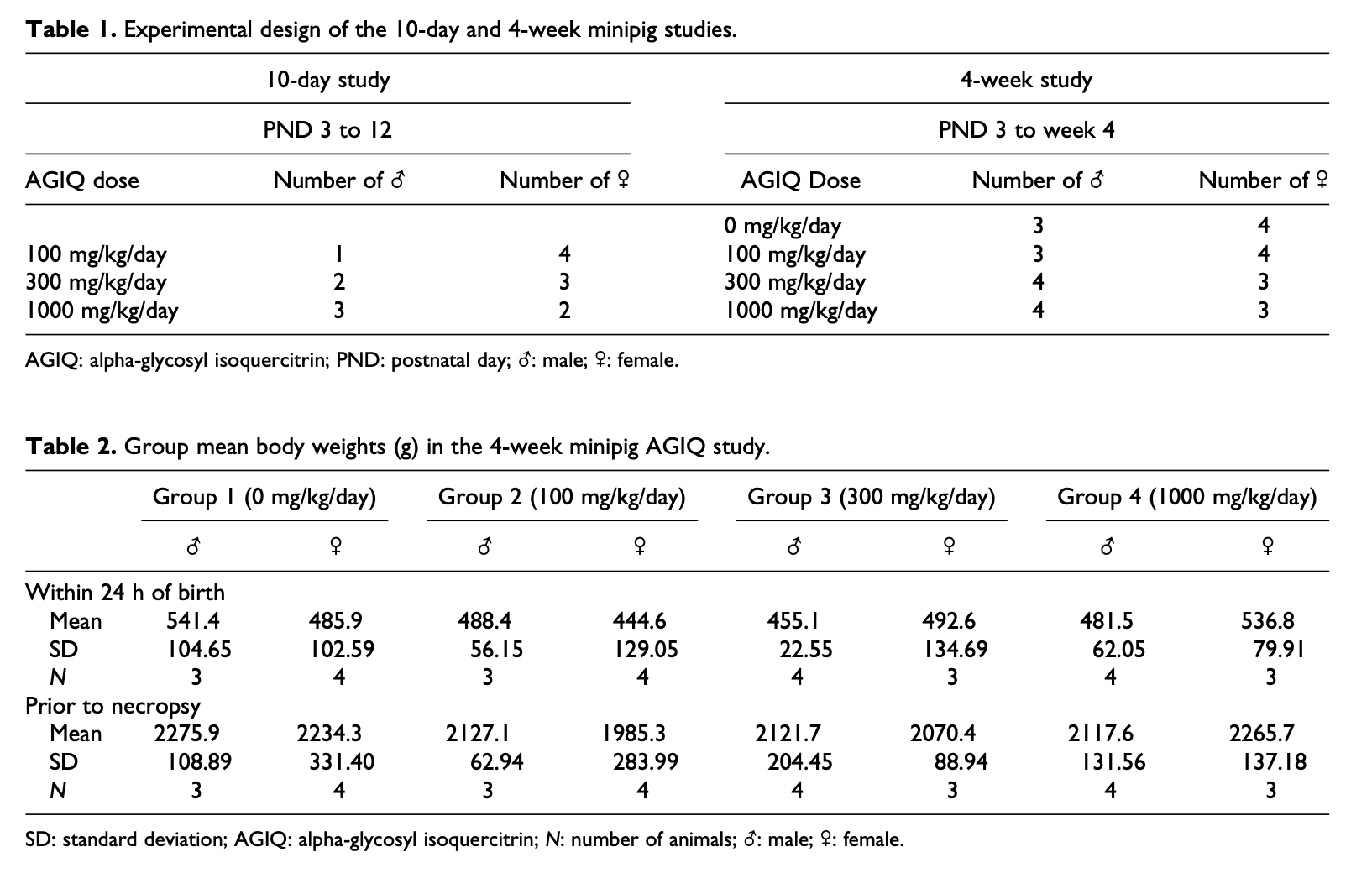
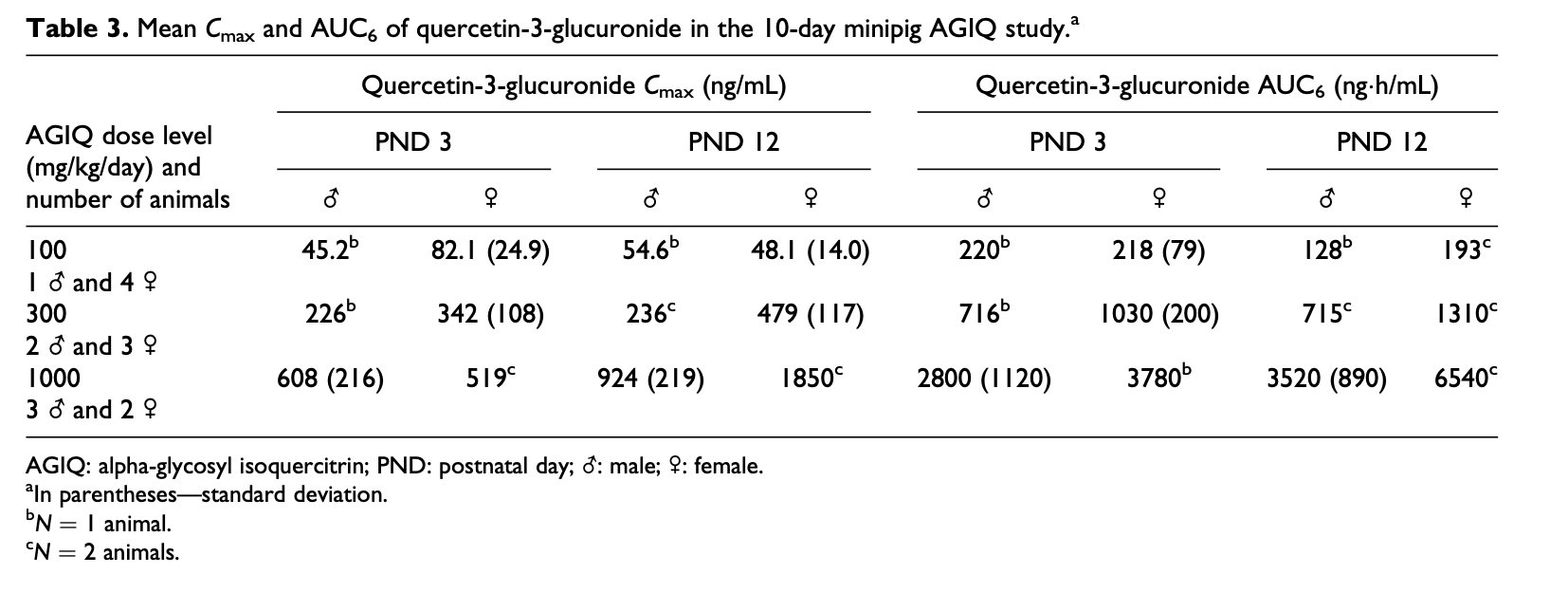
Microscopic examination did not reveal any changes related to treatment with AGIQ. Mixed inflammatory cell infiltrates were seen in the lungs of animals given 100, 300, or 1000 mg/kg/day. Subpleural alveolar fibrosis was also seen in two animals given 1000 mg/kg/day. As these findings are reported as background lesions seen in minipigs,30 they were considered to be of no relationship to treatment. Hepatocellular and sinusoidal/Kupffer cells pigments were seen in the liver of all animals (Figure 1(a)). Special staining with Perls Prussian Blue was conducted on the liver of representative animals and tested positive for iron deposits (Figure 1(b)). This finding was considered incidental and unrelated to AGIQ. Erythrocytosis/erythrophagocytosis and increased pigment containing macrophages were also seen in the lymph nodes of some animals.
Four-week study
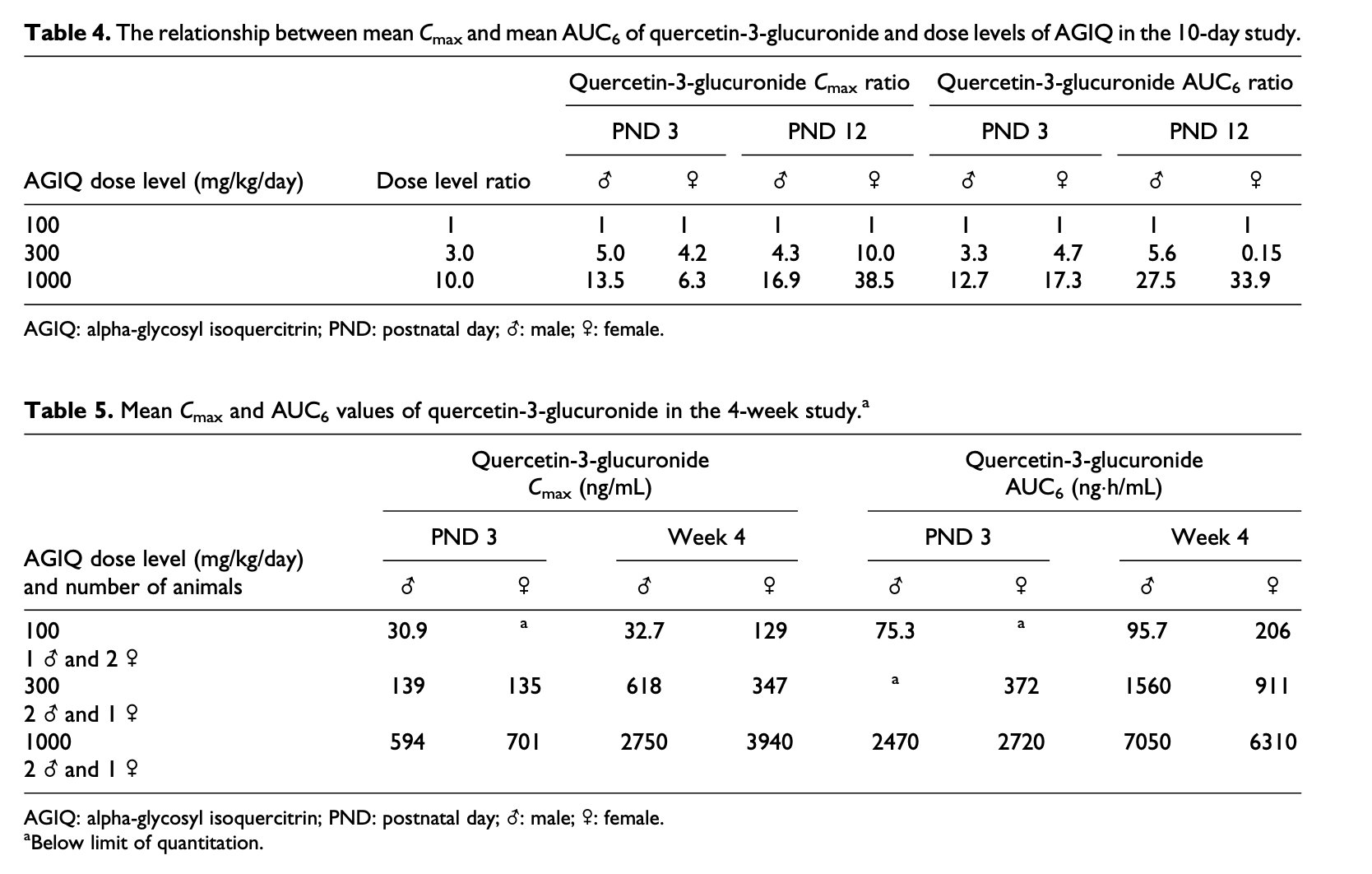
Yellow coloration of the femur and calvarium was seen in all animals treated with 1000 mg/kg/day and two males treated with 300 mg/kg/day (Figure 1(c)). However, microscopic examination did not reveal any changes related to treatment with AGIQ, and no histopathological correlation was seen for the yellowish coloration of the femur bones seen at necropsy in animals treated with 300 or 1000 mg/kg/day.
Hepatocellular and sinusoidal/Kupffer cells pigments were seen in the liver of all animals including controls, in similar staining intensity and distribution, as in the 10-day study, but with decreased amounts. Similar to the 10-day study, Perls Prussian Blue was positive for iron deposits (Figure 1(d)). In addition, increased pigment-containing macrophages were also seen in the lymph nodes of some animals including controls. These findings were considered incidental and unrelated to AGIQ due to a lack of dose-relationship and were considered likely to be iron deposits as seen in the liver.
Toxicokinetics
Ten-day study
With the exception of one female minipig plasma sample obtained at 24 h, all plasma levels of quercetin and isoquercitrin in the 100 mg/kg/day male and female groups were below the limit of quantitation (BLQ) on PNDs 3 and 12. Similarly, most samples in the 300 mg/kg/day groups for quercetin and isoquercitrin were also BLQ at both sampling intervals. At the 1000 mg/kg/day dose level, several minipig samples had detectable levels of quercetin and isoquercitrin, especially at the 0.5- and 6-h sampling intervals. In contrast, quercetin-3-glucuronide levels were quantifiable at most time points.
Quercetin
Mean maximum plasma concentrations (C max) and the mean areas under the plasma quercetin concentration–time curves estimated up to 6 h after the first daily dose (area under the curve [AUC6]) of quercetin results are summarized in Supplemental Table 4. Based on the available data in minipigs exposed to 1000 mg/kg/day, the maximum plasma concentration (T max) for quercetin was generally 0.5 h postdose and in the range of 0.5 to 6 h following the first daily dose, indicating absorption was generally rapid. Plasma concentrations of quercetin at 24 h after four daily dosings were only quantifiable on both PNDs 3 and 12 in one female, indicating that, in general at the dose of 1000 mg/kg/day, the minipigs were not continuously exposed to quantifiable concentrations of quercetin following four daily doses.
Isoquercitrin
C max and AUC6 of systemic exposure of preweaning female minipigs to isoquercitrin could only be assessed at the highest dose level (1000 mg/kg/day), where values in females on PND 3 were 1.6- to 2.3-fold higher, respectively, than in males (Supplemental Table 5). After repeated oral doses (PND 12), C max and AUC6 of systemic exposure of preweaning minipigs to isoquercitrin at the 1000 mg/kg/day dose level were generally lower than those values after a single dose (PND 3). The systemic isoquercitrin ratios, based on AUC6 values of PNDs 3 and 12, in one male minipig and one female minipig were 0.59 and 0.65, respectively, indicating that no saturation of the excretion process for isoquercitrin occurred in either of these two animals following repeated oral administration of AGIQ at the 1000 mg/kg/day dose level.
Quercetin-3-glucuronide
Mean maximum plasma concentrations (C max) of quercetin-3-glucuronide and the mean areas under the plasma quercetin-3-glucuronide concentration–time curves estimated up to 6 h after the first daily dose (AUC6) on PND 3 and PND 12 (days 1 and 10 of treatment) are summarized in Table 3. C max and AUC6 of systemic exposure of preweaning minipigs to quercetin-3-glucuronide on PND 3 generally increased approximately proportionately with increasing dose over the dose range 100 to 1000 mg/kg/day. However, systemic exposure of preweaning minipigs on PND 12 generally increased by more than the proportionate dose increment (Table 4). C max and AUC6 of systemic exposure of preweaning female minipigs to quercetin-3-glucuronide were generally higher than those indices in males and ranged from 15% lower to 2-fold higher than those of males.
After repeated oral doses (PND 12), C max and AUC6 of systemic exposure of preweaning minipigs to quercetin-3-glucuronide were higher than those values after a single dose (PND 3). The mean systemic quercetin-3-glucuronide ratios, based on AUC6 values of PNDs 3 and 12, were generally close to one in males and females at the 100 and 300 mg/kg/day dose levels and in males at the 1000 mg/kg/day dose level, but were greater than one at the 1000 mg/kg/day dose level in one female, indicating some saturation of quercetin-3-glucuronide excretion in that female after repeated oral administration of AGIQ at the 1000 mg/kg/day dose level (Supplemental Table 6).
Four-week study
The plasma concentrations of quercetin, isoquercitrin, and quercetin-3-glucuronide in the samples taken from the control animals were BLQ in all cases indicating that there was no quantifiable contamination with quercetin or its metabolites in these animals. All plasma samples from the 100 and 300 mg/kg/day groups were BLQ for quercetin and, with the exception of one male sampled on PND 3 (first day of treatment), plasma samples analyzed for isoquercitrin were also BLQ. Plasma concentrations of quercetin-3-glucuronide were present at most time points for all treated minipigs. The C max and AUC6 data of quercetin and isoquercitrin are summarized in Supplemental Tables 7 and 8, respectively. Quercetin-3-glucuronide data are summarized in Table 5.
Quercetin
The mean C max and AUC6 values of quercetin are provided in Supplemental Table 7. The time at which the maximum plasma concentration occurred (T max) was generally 0.5 h postdose, and in the range 0.5 to 2 h following the first daily dose, indicating that absorption was generally rapid. Although plasma concentrations of quercetin at 24 h after the four doses per day (C24) were quantifiable in two males receiving the 300 mg/kg/day dose level and in one male and one female receiving 1000 mg/kg/day on PND 3, quercetin C24 were BLQ in the remaining animals on PND 3 and in all animals at all dose levels on PND 30 (week 4), indicating that the animals were not continuously exposed to quantifiable concentrations of quercetin throughout the study following the four doses per day. After 4 weeks of repeated oral doses, C max and AUC6 of systemic exposure of preweaning minipigs to quercetin at the 1000 mg/kg/day dose level were lower than those values after a single dose on PND 3.
Isoquercitrin
The mean C max and AUC6 values of isoquercitrin are provided in Supplemental Table 8. Based on limited quantifiable data, the time at which the maximum plasma concentration of isoquercitrin occurred (T max) was generally 0.5 h postdose, and in the range 0.5 to 2 h following the first daily dose. Plasma concentrations of isoquercitrin at 24 h after the four doses per day (C24) were BLQ in all animals at all dose levels during week 4, indicating that the animals were not continuously exposed to quantifiable concentrations of isoquercitrin throughout the study following the four doses per day. After repeated oral doses (week 4), C max and AUC6 of systemic exposure of preweaning minipigs to isoquercitrin were lower than those values after a single dose on PND 3.
Quercetin-3-glucuronide
Plasma concentrations of quercetin-3-glucuronide at 24 h after the four doses per day (C24) were quantifiable in only two males receiving the 300 mg/kg/day dose and in all animals receiving 1000 mg/kg/day on both PND 3 and during week 4, indicating that these animals were continuously exposed to quantifiable concentrations of this metabolite throughout the study following the four doses per day. The mean C max and AUC6 values of quercetin-3-glucuronide are summarized in Table 5. The time at which the maximum plasma concentration of quercetin-3-glucuronide occurred (T max) was generally 0.5 h postdose, and in the range 0.5 to 2 h following the first daily dose. C max and AUC6 of systemic exposure of preweaning minipigs to quercetin-3-glucuronide was generally characterized by nonlinear (dose-dependent) kinetics over the dose range of 100 to 1000 mg/kg/day on both PND 3 and PND 30 (week 4). C max and AUC6 of systemic exposure of female preweaning minipigs to quercetin-3-glucuronide were generally similar to those indices of exposure in males except at the 100 mg/kg/day dose level during week 4 where C max and AUC6 were 3.9- and 2.2-fold higher, respectively, than in males. Based on AUC6 data limited to only one or two samples per dose level, there was some saturation of quercetin-3-glucuronide excretions after repeated oral administration of AGIQ in females receiving 300 mg/kg/day and in both sexes at the 1000 mg/kg/day dose level (Table 5).
Discussion
A preliminary 10-day study of orally administered AGIQ to preweaning Göttingen minipigs ruled out toxicity at a limit dose of 1000 mg/kg/day and provided initial evidence of systemic exposure to metabolite products of AGIQ. The AGIQ was provided as a yellow to yellow-orange powder, and after consumption resulted in yellow coloration of organs and secretions. In the main 4-week study, AGIQ consumption resulted in yellow coloration of the bones and was readily documented in the femur and calvarium. Similar yellow coloration of bones was also observed in a 90-day toxicity study in Sprague-Dawley rats,27 and in F344/DuCri rats during a 13-week oral toxicity and 4-week recovery study with AGIQ.31 In all cases, these color changes did not result in alteration of bone growth or in histological changes. In the present 4-week study, the consumption of the yellowish AGIQ also affected the color of secreted urine, with no effect on any of the urinalysis parameters.
In the 4-week study, there was a slight decrease in body weight gain in most treated groups when compared to controls over the duration of the study. However, there was a slight gain of weight in females given 1000 mg/kg/day when compared to controls. These changes are consistent with the high variability in individual body weights in preweaning minipigs of the age used in the study and, thus, are not considered an adverse response to treatment.
Preweaning minipigs require large amount of iron during their first days of life due to their rapid increase in weight. The iron is required mainly for myoglobin formation and erythropoiesis, and, if not supplied early and in appropriate amounts, the pigs may develop anemia which can be fatal. Therefore, all minipigs receive iron supplementations shortly after birth.32,33 In our study, the piglets received intramuscular injection of iron supplementation on PND 1 and 2. After injection, the iron can be found in histopathological sections in large amounts in organs rich in cells from the mononuclear phagocytic system, including the liver and the lymph nodes. Iron deposits can also be found occasionally in other organs, such as the reticulocytes of the bone marrow, adrenocortical sinuses, in the skeletal muscle (injection site), or perineural connective tissue and in the kidneys.33 Such findings are not considered adverse and emphasize the importance of proper knowledge of the normal histopathological findings that can be expected in newborn minipigs.34 Indeed, the amount of iron that was detected in the histological sections decreased between the 10-day study and the 4-week study, showing that its presence was transient and without any adverse effect on the animals.
The preliminary 10-day study confirmed doses for the 4-week study, indicating no adverse effects of treatment at up to a limit dose of 1000 mg/kg/day. While TK data were limited, there was evidence of exposure at the limit dose in both studies. Four times daily administration of AGIQ to preweaning minipigs from PND 3 to PND 30 resulted in rapid absorption with transient plasma concentrations of quercetin and isoquercitrin. The major metabolite of AGIQ, quercetin-3-glucuronide, showed a dose-related increase in plasma concentration that was greater than dose-proportional and with some evidence of saturation of excretion especially at 1000 mg/kg/day, indicating that the animals were continuously exposed to quantifiable concentrations of this metabolite throughout the study.
While quantifiable TK data were limited, there was sufficient evidence of systemic exposure to AGIQ at 1000 mg/kg/day in both 10-day and 4-week studies based on plasma levels of metabolites. Thus, the 10-day and 4-week toxicity studies in preweaning minipigs presented here provide strong evidence that oral administration of AGIQ is safe and does not show any adverse toxic effect in doses up to 1000 mg/kg/day. These results strengthen the positive safety profile of AGIQ as was also shown in the 90-day study in Sprague-Dawley rats.27
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AGIQ is a product of San-Ei Gen F.F.I., Inc. S Hayashi and M Koyanagi are San-Ei Gen F.F.I., Inc. employees. R Maronpot and A Nyska are paid consultants of San-Ei Gen F.F.I., Inc. Y Ramot, N Dias, D Cameron, and S Eniola declare no conflict of interests.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by San-Ei Gen F.F.I., Inc., Osaka, Japan.
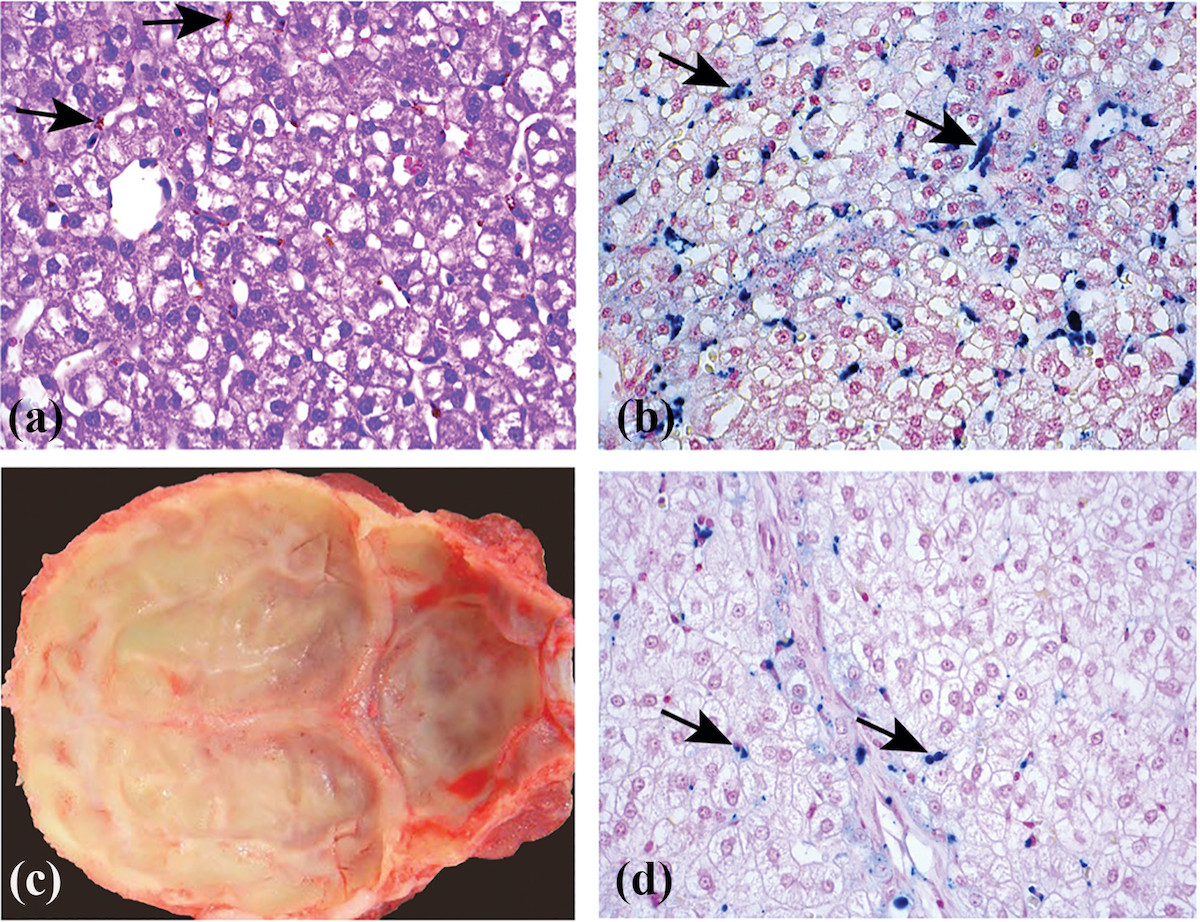
Figure 1. (a) Section of the liver of a minipig treated for 10 days with 1000 mg/kg/day of AGIQ stained by H&E. Arrows identify Kupffer cells containing brownish pigment. The pigmentation is related to administration of iron formulation to newborn piglets and was considered minor, and decreased in amount within weeks, as demonstrated in the 1-month study (see (d)). ×40. (b) Section of the liver of a minipig treated for 10 days with 1000 mg/kg/day of AGIQ stained by Prussian Blue for iron. Arrows identify Kupffer cells containing positively reacting iron pigment. The pigmentation was scored as minor and decreased in amount within weeks as demonstrated in the 1-month study (see (d)). ×40. (c) Macroscopic view showing yellow coloration of the calvarium visceral surface from a minipig treated for 4-weeks with 1000 mg/kg/day of AGIQ. No abnormal histopathological findings were present in bone sections. (d) Section of the liver of a female minipig treated for 30 days with 1000 mg/kg/day of AGIQ and stained by Prussian Blue for iron. Arrows identify Kupffer cells containing positively reacting pigment. The pigmentation was minor in amount and was reduced in amount over weeks, compared to the 10 days study (see (b)). ×40. AGIQ: alpha-glycosyl isoquercitrin; H&E: hematoxylin and eosin.
References
1. Li, R, Yuan, C, Dong, C. In vivo antioxidative effect of isoquercitrin on cadmium-induced oxidative damage to mouse liver and kidney. Naunyn Schmiedeb Arch Pharmacol 2011; 383: 437–445.
2. Rogerio, AP, Kanashiro, A, Fontanari, C. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res 2007; 56: 402–408.
3. Amado, NG, Cerqueira, DM, Menezes, FS. Isoquercitrin isolated from Hyptis fasciculata reduces glioblastoma cell proliferation and changes beta-catenin cellular localization. Anticancer Drugs 2009; 20: 543–552.
4. Huang, XL, He, Y, Ji, LL. Hepatoprotective potential of isoquercitrin against type 2 diabetes-induced hepatic injury in rats. Oncotarget 2017; 8: 101545–101559.
5. Guo, XD, Zhang, DY, Gao, XJ. Quercetin and quercetin-3-O-glucuronide are equally effective in ameliorating endothelial insulin resistance through inhibition of reactive oxygen species-associated inflammation. Mol Nutr Food Res 2013; 57: 1037–1045.
6. Hassan, W, Rongyin, G, Daoud, A. Reduced oxidative stress contributes to the lipid lowering effects of isoquercitrin in free fatty acids induced hepatocytes. Oxid Med Cell Longev 2014; 2014: 313602.
7. Manach, C, Morand, C, Demigne, C. Bioavailability of rutin and quercetin in rats. FEBS Lett 1997; 409: 12–16.
8. Manach, C, Williamson, G, Morand, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005; 81: 230S–242S.
9. You, HJ, Ahn, HJ, Ji, GE. Transformation of rutin to antiproliferative quercetin-3-glucoside by Aspergillus niger. J Agric Food Chem 2010; 58: 10886–10892.
10. Murota, K, Matsuda, N, Kashino, Y. Alpha-oligoglucosylation of a sugar moiety enhances the bioavailability of quercetin glucosides in humans. Arch Biochem Biophys 2010; 501: 91–97.
11. Fujii, Y, Kimura, M, Ishii, Y. Effect of enzymatically modified isoquercitrin on preneoplastic liver cell lesions induced by thioacetamide promotion in a two-stage hepatocarcinogenesis model using rats. Toxicology 2013; 305: 30–40.
12. Hara, S, Morita, R, Ogawa, T. Tumor suppression effects of bilberry extracts and enzymatically modified isoquercitrin in early preneoplastic liver cell lesions induced by piperonyl butoxide promotion in a two-stage rat hepatocarcinogenesis model. Exp Toxicol Pathol 2014; 66: 225–234.
13. Kangawa, Y, Yoshida, T, Abe, H. Anti-inflammatory effects of the selective phosphodiesterase 3 inhibitor, cilostazol, and antioxidants, enzymatically-modified isoquercitrin and alpha-lipoic acid, reduce dextran sulphate sodium-induced colorectal mucosal injury in mice. Exp Toxicol Pathol 2017; 69: 179–186.
14. Kangawa, Y, Yoshida, T, Maruyama, K. Cilostazol and enzymatically modified isoquercitrin attenuate experimental colitis and colon cancer in mice by inhibiting cell proliferation and inflammation. Food Chem Toxicol 2017; 100: 103–114.
15. Kimura, M, Fujii, Y, Yamamoto, R. Involvement of multiple cell cycle aberrations in early preneoplastic liver cell lesions by tumor promotion with thioacetamide in a two-stage rat hepatocarcinogenesis model. Exp Toxicol Pathol 2013; 65: 979–988.
16. Morita, R, Shimamoto, K, Ishii, Y. Suppressive effect of enzymatically modified isoquercitrin on phenobarbital-induced liver tumor promotion in rats. Arch Toxicol 2011; 85: 1475–1484.
17. Nishimura, J, Saegusa, Y, Dewa, Y. Antioxidant enzymatically modified isoquercitrin or melatonin supplementation reduces oxidative stress-mediated hepatocellular tumor promotion of oxfendazole in rats. Arch Toxicol 2010; 84: 143–153.
18. Shimada, Y, Dewa, Y, Ichimura, R. Antioxidant enzymatically modified isoquercitrin suppresses the development of liver preneoplastic lesions in rats induced by beta-naphthoflavone. Toxicology 2010; 268: 213–218.
19. Yoshida, T, Murayama, H, Kawashima, M. Apocynin and enzymatically modified isoquercitrin suppress the expression of a NADPH oxidase subunit p22phox in steatosis-related preneoplastic liver foci of rats. Exp Toxicol Pathol 2017; 69: 9–16.
20. Ministry of Health, Labour and Welfare (MHLW) . List of existing food additives as of November 30, 2017, https://www.ffcr.or.jp/tenka/list/post-12.html (accessed 29 May 2019).
21. FDA , Agency Response Letter GRAS Notice No. GRN00220 [alpha-glycosyl isoquercitrin]. US Food and Drug Administration Center for Food Safety and Applied Nutrition, 2007.
22. Engen, A, Maeda, J, Wozniak, DE. Induction of cytotoxic and genotoxic responses by natural and novel quercetin glycosides. Mutat Res Genet Toxicol Environ Mutagen 2015; 784-785: 15–22.
23. Salim, EI, Kaneko, M, Wanibuchi, H. Lack of carcinogenicity of enzymatically modified isoquercitrin in F344/DuCrj rats. Food Chem Toxicol 2004; 42: 1949–1969.
24. Valentova, K, Vrba, J, Bancirova, M. Isoquercitrin: pharmacology, toxicology, and metabolism. Food Chem Toxicol 2014; 68: 267–282.
25. Constable, A, Mahadevan, B, Pressman, P. An integrated approach to the safety assessment of food additives in early life. Toxicol Res App 2017; 1: 1–26.
26. Guilloteau, P, Zabielski, R, Hammon, HM. Nutritional programming of gastrointestinal tract development. Is the pig a good model for man? Nutr Res Rev 2010; 23: 4–22.
27. Nyska, A, Hayashi, SM, Koyanagi, M. Ninety-day toxicity and single-dose toxicokinetics study of alpha-glycosyl isoquercitrin in Sprague-Dawley rats. Food Chem Toxicol 2016; 97: 354–366.
28. Hobbs, CA, Koyanagi, M, Swartz, C. Comprehensive evaluation of the flavonol anti-oxidants, alpha-glycosyl isoquercitrin and isoquercitrin, for genotoxic potential. Food Chem Toxicol 2018; 113: 218–227.
29. Shackelford, C, Long, G, Wolf, J. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol 2002; 30: 93–96.
30. Jeppesen, G, Skydsgaard, M. Spontaneous background pathology in Göttingen minipigs. Toxicol Pathol 2015 43: 257–266.
31. Tamano, S, Hatahara, Y, Sano, M. 13-Week oral toxicity and 4-week recovery study of enzymatically modified isoquercitrin in F344/DuCrj rats. Jpn J Food Chem 2001; 8: 161–167.
32. Ellegaard . Liver in the notes on the histology of the Göttingen minipigs. Ellegaard Göttingen Minipigs A/S 2008; 18–20.
33. Rinke, M . How clean is a mini-pig? Impressions and suggestions of a pathologist working in the field of toxicology. Pharmacol Toxicol 1997; 80(suppl 2): 16–22.
34. Ramot, Y, Weber, K, Moreno Lobato, B. Trauma as a cause for hepatopathy in newborn Gottingen minipigs. Toxicol Pathol 2016; 44: 1123–1127.