Yuval Ramot, Yael S. Schiffenbauer, Robert Maronpot, and Abraham Nyska
View as PDFDrug development is a lengthy and highly expensive endeavor involving high risk and uncertainty. Animal studies are a crucial part of this process, both for the evaluation of drug efficacy and for drug safety assessment. For financial and ethical reasons, it is important to find ways to reduce the number of study animals needed in preclinical testing. Since preclinical assessments should be done in a timely manner and in a strictly regulated environment, the question of how to optimize the process so that resources may be prudently allocated is a major concern for all drug developers (Jekunen, 2014). If data gathering is modified by adding imaging to preclinical studies, the drug development process can become more efficient with an added benefit of providing an approach that may be translatable to the clinical setting. Imaging technologies have made tremendous advances in the last 3–4 decades (Ying and Monticello, 2006) and can be readily used to facilitate drug development. In addition to X-ray and ultrasound, preclinical imaging modalities available now for researchers include magnetic resonance imaging (MRI), microcomputed tomography, micro positron emission tomography and single-photon emission computed tomography and in vivo optical imaging, while additional new and combinatory technologies continue to develop (Ettlin, 2013; Ying and Monticello, 2006).
Magnetic Resonance Imaging In Drug Development And Toxicity Studies
Magnetic resonance imaging is one of the main technologies that holds great promise for preclinical research and animal studies in support of drug development and safety assessment, and, indeed, for use in general investigative biomedical research. Laboratory animal MRI has been utilized in toxicologic pathology for almost 30 years (Delnomdedieu et al., 1996; Johnson and Maronpot, 1989; Maronpot et al., 2004) and is an excellent modality for noninvasive in vivo, as well as ex vivo, imaging due to its high soft tissue contrast and spatial resolution. It allows sensitive detection of pathologic changes in soft tissues, provides quantitative three-dimensional data, and its use in longitudinal studies allows noninvasive monitoring of the genesis, progression, and regression of chemically induced changes (Dixon et al., 1988). By using MRI there is reduced need, and sometimes no need, for interim sacrifice as the same animal can be repeatedly and sequentially imaged over time, and can even serve as its own control when imaged before start of treatment.
The use of MRI in animal studies has also set the stage for the development of the new concept of magnetic resonance histology (MRH) (Johnson et al., 1993). MRH is the use of MR imaging on formalin-fixed tissues for high resolution characterization of tissue structure (Johnson et al., 2002). It is a highly valuable complementary adjunct to conventional histopathology, as it permits a thorough examination to be performed through multiple digital slices of an entire organ, while leaving the formalin-fixed specimen intact for subsequent definitive conventional diagnostic histopathology.
The Introduction Of Compact, Self-contained MRI Systems
While the advantages of using MRI in preclinical testing are evident, it is still underutilized and widespread adoption of MRI in preclinical investigations has been hampered by the high purchase price, the expense of siting and installation, and operating and maintenance costs of conventional superconducting MRI systems. In addition, there are significant safety concerns, infrastructure, and logistical issues associated with use of superconducting MRI systems. Consequently, use of MRI imaging in preclinical safety assessment studies has previously been limited to large research centers that could afford to acquire and maintain superconducting MRI equipment as well as to employ educated and trained staff to operate these expensive superconducting MRI magnets.
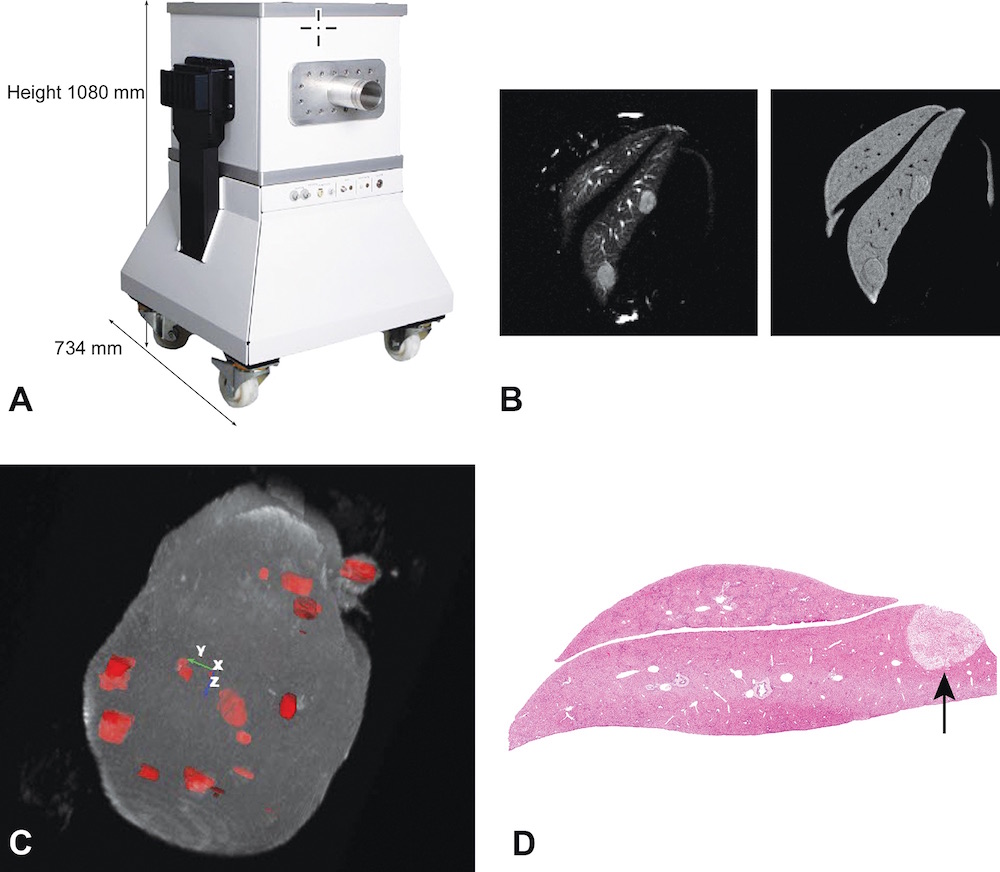
This situation has changed with the introduction of a portable, relatively inexpensive, self-contained, and self-shielded compact MRI system (Figure 1A) (Tempel-Brami et al., 2015). This new MRI imaging system can be placed in any laboratory or research facility without requiring specially shielded rooms, cryogens or coolants, or a dedicated electrical or plumbing supply. The portability of the compact MRI imaging equipment allows it to be easily moved into an animal room so that animals need not be removed from the animal facility. The ease of use of compact MRI equipment provides an opportunity for toxicologists and pathologists to obtain diagnostic-quality in vivo MRI and ex vivo MRH images of experimental rodents and tissue samples, thereby greatly enhancing conventional preclinical studies. Basically, the convenience of this type of MRI facilitates the development and use of rodent models of human disease without the cost, complexity, infrastructure, and safety issues traditionally associated with superconducting MRI systems (Geninatti-Crich et al., 2011; Schmid et al., 2013). Table 1 provides a comparison between compact MRI systems and traditional superconducting MRI systems.
A, View of a high performance compact MRI system. B, Two-dimensional views of the same focal liver lesions using different MRI image acquisition settings. C, MRI rendering and segmentation of a whole liver showing multiple focal lesions developing in an Mdr −/− KO mouse. D, Hematoxylin and eosin-stained section of one of the focal lesions in the liver of the Mdr −/− KO mouse.
A Comparison Between the Main Characteristics of Compact MRI and Traditional Superconducting MRI Systems
| Permanent Magnet Compact MRI | Traditional Superconducting MRI | |
|---|---|---|
| Size | Compact | Not compact |
| Field strength | 1 T | 4.7–9.4 T |
| Resolution | Limited to 60 microns ex vivo and 100 microns in vivo | Down to 20 microns ex vivo and 30 microns in vivo |
| Scan time | Typical 5 min for in vivo | At least 3 times faster |
| Cryogenic operation of magnet | No | Yes |
| Price | Low (starts from 300K US$) | At least 3 times more |
| Upfront site preparations | Negligible | Significant |
| No dedicated power supply, no RF shielded room, no cryogens | Infrastructure adaptations required (shielded/isolated room/water cooling/3-phase electricity/substantial HVAC) | |
| Safety | Very Safe | Restricted |
| Practically no fringe field, allowing no restrictions with magnet location | Special attention is required. May have extensive 5 Gauss stray field | |
| Siting and moving flexibility | Flexible | Limited flexibility |
| The instrument is wheeled and can be easily moved | Support infrastructure may make it problematic to move | |
| Animal handling for imaging | Instrument can be moved into animal facility | Animals must be removed from animal facility for imaging |
| Electricity and water cooling | Single-phase power outlet | Three Phase electricity |
| No water cooling required | Cryogenic infrastructure; compressor and cryogens | |
| Ongoing maintenance considerations and running costs | Negligible | Considerable |
| Electronics can be switched off overnight for zero energy costs | System is ON 24/7. Cryogenic infrastructure must be periodically serviced | |
| System operation | Routine operation, high throughput. Behind the barrier operation | Often requires MRI expertise |
| Advanced MRI protocols | Limited | Available |
| Acoustic noise | Negligible acoustic noise | Substantial (may require acoustic shielding) |
HVAC, heating, ventilating, and air-conditioning.
In the system described by Tempel-Brami et al. (2015), in vivo images were acquired with acquisition times ranging from 2 to 10 min and ex vivo MRH images on fixed tissue were batch loaded for automatic image acquisition. The short acquisition times and automatic image acquisition on fixed specimens allow for a degree of high-throughput and practicality not achievable with conventional superconducting MRI systems. The associated operating software of these new self-contained MRI systems permits operation and image acquisition by regular laboratory staff without requiring onsite specialized expertise. After proper instruction and training, laboratory toxicologic pathologists and laboratory animal veterinarians can do the interpretation and evaluation of acquired images. Multiple vendors provide compact MRI systems, including Aspect Imaging (www.aspectimaging.com), Bruker (www.bruker.com) and MR solutions (www.mrsolutions.com).
Applications Of MRI Imaging In Toxicology Studies
Magnetic resonance imaging has been utilized in several proof-of-principle studies, encompassing a wide range of applications, including renal toxicity, neurotoxicity, safety evaluation of stem cells, quantitating pulmonary fibrosis, and in rodent cancer model studies. In these and other studies, compact MRI systems have provided images pertinent to safety and/or efficacy assessments and have generated quantitative information on tissue changes based on size, signal intensity and relaxation maps (T1 and T2) that may serve as accurate MRI endpoints and are intrinsically translatable to clinical settings.
One of the principal objectives of the regulatory requirements for preclinical safety evaluations is to ensure that the potential toxicity of new drugs and chemicals is adequately tested and to assess recovery potential that may be translatable to exposed humans (Dorato and Engelhardt, 2005). Using sequential MRI imaging in the same animal, in vivo and ex vivo MRI was shown effective in identifying toxic alterations in the renal cortex and medulla and to follow the recovery during tissue repair (Tempel-Brami et al., 2015). Identifying the location of neurotoxic effects in the central nervous system is even more challenging since the brain is a nonuniform organ and difficult to adequately examine by conventional histopathology (Hanig et al., 2014). The Society of Toxicologic Pathology (STP) has recently recommended increasing brain sampling to seven or eight sections to enhance the sensitivity of detecting neurotoxic effects (Bolon et al., 2006), but despite the extra time and resources needed for this evaluation, there remains a consensus that even the addition of more trimmed areas for histopathological examination does not suffice to cover the entire brain. Therefore, many important regions of the brain will not be evaluated in routine preclinical and toxicology studies. MRI analysis of a whole fixed brain, however, provides a more comprehensive evaluation, enabling affected regions to be accurately identified, and thereby identifying appropriate regions to be sampled for conventional histopathology (Ramot et al., 2017; Taketa et al., 2015). Indeed, a validation study recently performed by the US Food and Drug Administration (FDA) concluded that full-brain MRI could significantly improve detection of neurotoxicity caused by new compounds (Hanig et al., 2014). Thus, following appropriate validation studies, MRI could easily serve as a biomarker and quantitative endpoint for the detection of potential neurotoxic compounds.
One of the novel proposed applications of stem cells is for curing neurodegenerative diseases (Nakamura et al., 2015; Oh et al., 2015; Villanova and Bach, 2015), but the safe use of stem cell therapy involves their carcinogenic potential, such as teratoma formation (Berkowitz et al., 2016; Cunningham et al., 2012). Our recent preclinical investigations have shown that MRI can be used for time-course (longitudinal) observations of the carcinogenic potential of novel stem cells intended for clinical use. We have shown both in vivo and ex vivo MRI to be effective in accurately detecting the onset, localization and growth of teratomas in the brain and spinal cord of experimental animals following injection of human stem cells (Ramot et al., 2017).
In vivo and ex vivo MRI in combination with histopathology enables a comprehensive study of the local tolerance of implanted devices. A particularly useful application of MRI is in monitoring the degradation and reabsorption rate of bio-degradable implants. In addition, MRI imaging allows the quantitation and morphometric parameters of tissue inflammatory reactions to implanted devices (Nyska et al., 2014). Other investigators have shown the advantages of using bimodal MRI/fluorescence imaging in providing important qualitative and quantitative information when investigating the in vivo material stability, integration, and resorption as related to the therapeutic potential of an implanted material (Berdichevski et al., 2015).
One of the main challenges in testing new drug infusions and subcutaneous formulations is selecting compounds that cause the least degree of local irritation (Nyska et al., 1994; Ramot et al., 2012, 2015; Tanner and Marks, 2008). Ex vivo MRI has been used to define the location and to quantify the extent of subcutaneous necrosis and inflammation caused by different subcutaneously administered test materials, thereby identifying the relative irritancy of different injected formulations (Nyska et al., 2014; Tempel-Brami et al., 2015).
There are other applications in experimental and toxicology studies that have been shown to take advantage of the ability of MRI to scan the entire volume of an animal or a specific organ, enabling the investigator to locate, count, and measure the size of each lesion or area of tissue damage. Conventional histopathology would require thousands of serial sections and subsequent morphometric measurements to achieve what MRI can provide in a relatively short scan time. It is clear that definitive diagnosis of individual lesions requires conventional histopathology. What the MRI images provide in addition to the quantitative data is a guide to where tissues should be sampled for histopathological diagnosis. In this regard, in vivo and ex vivo MRI can be applied to anticancer therapy studies using experimental brain, mammary gland and ovarian tumor models, in pulmonary fibrosis models, and in locating and measuring the volume of preneoplastic changes in liver and kidney carcinogenesis models (Figs. 1B–D) (Tempel-Brami et al., 2015).
Regulatory Aspects For The Use Of Mri Imaging In Preclinical Studies
The use of MRI is currently not good laboratory practice (GLP) compliant. Validation of certain nonroutine study endpoints, such as MRI, is currently challenging, and GLP exceptions for such study components will continue to be necessary. Nevertheless, we believe that imaging, as a component of toxicity studies and safety evaluation as well as an adjunct to conventional histopathology, allows for better science. Imaging also provides three-dimensional quantitative data for tissue changes that cannot be easily achieved using traditional pathology approaches. Since imaging in safety evaluation studies is inherently translatable to clinical settings, we believe it is a desirable component of regulatory submissions. Indeed, informal shared experience from colleagues involved in drug development suggests that historically the FDA has accepted the submission of imaging reports, when included as non-GLP components within a GLP study (similar to immunohistochemistry endpoints, electron microscopy, or other special assessments using nonvalidated assays), as long as the imaging data acquisition was demonstrated to be scientifically rigorous with appropriate controls.
In conclusion, the availability of compact, self-contained MRI systems now permits a much more feasible, cost-effective, and practical use of MRI in biomedical research and preclinical drug development as an alternative to the use of superconducting MRI systems. We believe that data will accumulate from examples such as those mentioned above that will enrich the science that can be supported by this technology. With the growing use of MRI in preclinical studies, regulatory agencies will accept and provide guidance on use of this and other imaging modalities.
Acknowledgments
We thank Dr Rinat Abramovitch, Hadassah Hebrew University Medical Center, Jerusalem, Israel, for providing the Mdr (−/−) mouse model presented in this article.
Y.S.S. is an employee of Aspect Imaging. A.N. serves as a consultant for Aspect Imaging.
References
Berdichevski A.Simaan Yameen H.Dafni H.Neeman M.Seliktar D. (2015). Using bimodal MRI/fluorescence imaging to identify host angiogenic response to implants. Proc. Natl. Acad. Sci. U. S. A . 112, 5147–5152.
Berkowitz A. L.Miller M. B.Mir S. A.Cagney D.Chavakula V.Guleria I.Aizer A.Ligon K. L.Chi J. H. (2016). Glioproliferative lesion of the spinal cord as a complication of “stem-cell tourism”. New Engl. J. Med . 375, 196–198.
Bolon B.Garman R.Jensen K.Krinke G.Stuart B. (2006). A ‘best practices’ approach to neuropathologic assessment in developmental neurotoxicity testing–for today. Toxicol. Pathol . 34, 296–313.
Cunningham J. J.Ulbright T. M.Pera M. F.Looijenga L. H. (2012). Lessons from human teratomas to guide development of safe stem cell therapies. Nat. Biotechnol . 30, 849–857.
Delnomdedieu M.Hedlund L. W.Johnson G. A.Maronpot R. R. (1996). Magnetic resonance microscopy–a new tool for the toxicologic pathologist. Toxicol. Pathol . 24, 36–44.
Dixon D.Johnson G. A.Cofer G. P.Hedlund L. W.Maronpot R. R. (1988). Magnetic resonance imaging (MRI): A new tool in experimental toxicologic pathology. Toxicol. Pathol . 16, 386–391.
Dorato M. A.Engelhardt J. A. (2005). The no-observed-adverse-effect-level in drug safety evaluations: Use, issues, and definition(s). Regul. Toxicol. Pharmacol . 42, 265–274.
Ettlin R. A. (2013). Toxicologic pathology in the 21st century. Toxicol. Pathol . 41, 689–708.
Geninatti-Crich S.Szabo I.Alberti D.Longo D.Aime S. (2011). MRI of cells and mice at 1 and 7 Tesla with Gd-targeting agents: When the low field is better!. Contrast Media Mol. Imaging 6, 421–425.
Hanig J.Paule M. G.Ramu J.Schmued L.Konak T.Chigurupati S.Slikker W.JrSarkar S.Liachenko S. (2014). The use of MRI to assist the section selections for classical pathology assessment of neurotoxicity. Regul. Toxicol. Pharmacol . 70, 641–647.
Jekunen A. (2014). Decision-making in product portfolios of pharmaceutical research and development–managing streams of innovation in highly regulated markets. Drug Des. Devel. Ther . 8, 2009–2016.
Johnson G. A.Benveniste H.Black R. D.Hedlund L. W.Maronpot R. R.Smith B. R. (1993). Histology by magnetic resonance microscopy. Magn. Reson. Q . 9, 1–30.
Johnson G. A.Cofer G. P.Fubara B.Gewalt S. L.Hedlund L. W.Maronpot R. R. (2002). Magnetic resonance histology for morphologic phenotyping. J. Magn. Reson. Imaging 16, 423–429.
Johnson G. A.Maronpot R. R. (1989). Magnetic resonance microscopy of chemically-induced liver foci. Toxicol. Pathol . 17, 613–616.
Maronpot R. R.Sills R. C.Johnson G. A. (2004). Applications of magnetic resonance microscopy. Toxicol. Pathol . 32(Suppl. 2), 42–48.
Nakamura K.Mieda T.Suto N.Matsuura S.Hirai H. (2015). Mesenchymal stem cells as a potential therapeutic tool for spinocerebellar ataxia. Cerebellum 14, 165–170.
Nyska A.Schiffenbauer Y. S.Brami C. T.Ramot Y. (2014). Histopathology of biodegradable polymers: Challenges in interpretation and the use of a novel compact MRI for biocompatibility evaluation. Polymer. Adv. Tech . 25, 461–467.
Nyska A.Skolnick M.Ziv G.Gulkarov A. (1994). Correlation of injection site damage and serum creatine kinase activity in turkeys following intramuscular and subcutaneous administration of norfloxacin nicotinate. Avian Pathol . 23, 671–682.
Oh K. W.Moon C.Kim H. Y.Oh S. I.Park J.Lee J. H.Chang I. Y.Kim K. S.Kim S. H. (2015). Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl. Med . 4, 590–597.
Ramot Y.Rosenstock M.Klinger E.Bursztyn D.Nyska A.Shinar D. M. (2012). Comparative long-term preclinical safety evaluation of two glatiramoid compounds (glatiramer Acetate, Copaxone(R), and TV-5010, protiramer) in rats and monkeys. Toxicol. Pathol . 40, 40–54.
Ramot Y.Schiffenbauer Y. S.Amouyal N.Ezov N.Steiner M.Izrael M.Lavon N.Hasson A.Revel M.Nyska A. (2017). Compact MRI for the detection of teratoma development following intrathecal human embryonic stem cell injection in NOD-SCID mice. Neurotoxicology 59, 27–32.
Ramot Y.Touitou D.Levin G.Ickowicz D. E.Zada M. H.Abbas R.Yankelson L.Domb A. J.Nyska A. (2015). Interspecies differences in reaction to a biodegradable subcutaneous tissue filler: Severe inflammatory granulomatous reaction in the Sinclair minipig. Toxicol. Pathol . 43, 267–271.
Schmid A.Schmitz J.Mannheim J. G.Maier F. C.Fuchs K.Wehrl H. F.Pichler B. J. (2013). Feasibility of sequential PET/MRI using a state-of-the-art small animal PET and a 1 T benchtop MRI. Mol. Imaging Biol . 15, 155–165.
Taketa Y.Shiotani M.Tsuru Y.Kotani S.Osada Y.Fukushima T.Inomata A.Hosokawa S. (2015). Application of a compact magnetic resonance imaging system for toxicologic pathology: Evaluation of lithium-pilocarpine-induced rat brain lesions. J. Toxicol. Pathol . 28, 217–224.
Tanner T.Marks R. (2008). Delivering drugs by the transdermal route: Review and comment. Skin Res. Technol . 14, 249–260.
Tempel-Brami C.Schiffenbauer Y. S.Nyska A.Ezov N.Spector I.Abramovitch R.Maronpot R. R. (2015). Practical applications of in vivo and ex vivo MRI in toxicologic pathology using a novel high-performance compact MRI system. Toxicol. Pathol . 43, 633–650.
Villanova M.Bach J. R. (2015). Allogeneic mesenchymal stem cell therapy outcomes for three patients with spinal muscular atrophy type 1. Am. J. Phys. Med. Rehabil . 94, 410–415.
Ying X.Monticello T. M. (2006). Modern imaging technologies in toxicologic pathology: An overview. Toxicol. Pathol . 34, 815–826.