Immunohistochemical (IHC) staining for Casein Kinase 2 alpha (CK2a), cyclin-dependent kinase inhibitor 1B (p27Kip1), and heat shock protein 90 (HSP90) was performed on four levels of decalcified rat nasal cavity from four male and three female controls following nose-only exposure to ambient air for 5 days. Successful staining with negligible background artifact was obtained on multiple epithelial and neuroepithelial soft tissues, without antigen retrieval. Independent optimization was needed for each of the three antibodies. A major challenge in this project was maintaining tissue adherence to the slides during tissue incubations. This report documents immunolocalization of CK2a, p27Kip1, and HSP90 in respiratory and olfactory tissue of the rat nose.
Keywords: CK2a, Decalcification, HSP90, Immunohistochemistry, Olfactory epithelium, p27Kip1, Rat nasal tissue, Respiratory epithelium
Introduction
The challenges associated with obtaining high quality immunohistochemistry (IHC) include variables such as tissue fixation, antibody specificity, antigen retrieval, reagent dilution, and incubation times. Additional challenges are introduced in performing IHC on rodent noses that were previously archived and subsequently decalcified. Decalcified rodent nose is difficult to section because the incisor and molar teeth retain a degree of hardness and easily develop tissue folds. The bony tissue structures have trouble adhering to the glass slide and often lift off during the numerous incubation steps of the staining process. Such is the case in performing IHC on decalcified sections of the noses of rats and mice. Nasal sections from small rodents contain a range of tissue types, from delicate ciliated respiratory epithelium to cartilage to dental enamel. These animals are used in testing drugs, pesticides, water disinfection byproducts, and other chemicals involved in environmental exposures in order to identify potential hazards to human health.
High throughput microarray and proteomic technologies generate hundreds to thousands of expression profiles. In analyzing these data it is most practical to initially focus on changes most clearly up- or down-regulated. Three immunohistochemical assays, CK2a, p27Kip1, and HSP90, were selected based on the most promising data obtained from proteomic analysis of nose tissue lysates in tobacco smoke-exposed versus control rats. Since the proteomic data from the smoke-exposed rats did not appropriately confirm the IHC for the selected proteins, the study was restricted to controls.
CK2a is a highly conserved serine/threonine kinase that phosphorylates substrates involved in a wide variety of biological and pathological processes. It shows both cytoplasmic and nuclear localization by IHC and plays a critical role in the cell cycle, influencing cell proliferation and cellular senescence. P27Kip1, a member of the kinase inhibitory protein family, functions as a critical switch in growth arrest, prompting subsequent cellular differentiation or apoptosis. It, too, can be localized in both the nucleus and the cytoplasm.5 Finally, HSP90 is highly abundant in eukaryotic organisms, accounting for 1– 2% of normal cellular protein. It is involved in folding, translocation, and degradation of intracellular protein in both normal and stress conditions, is induced by environmental stress, is involved in oxidative stress defense, and can trigger innate and adaptive immunity.
The purposes of this paper are to present efforts to obtain useful IHC on air-exposed control rats from this study and to show some of the results in the different cell types of the nose.
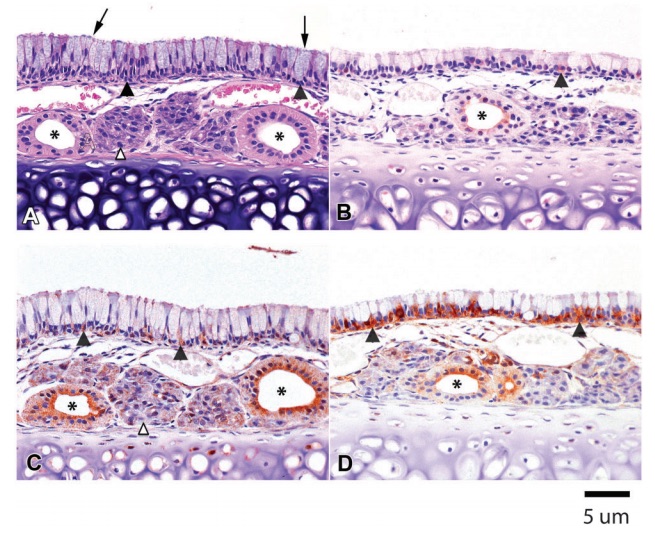
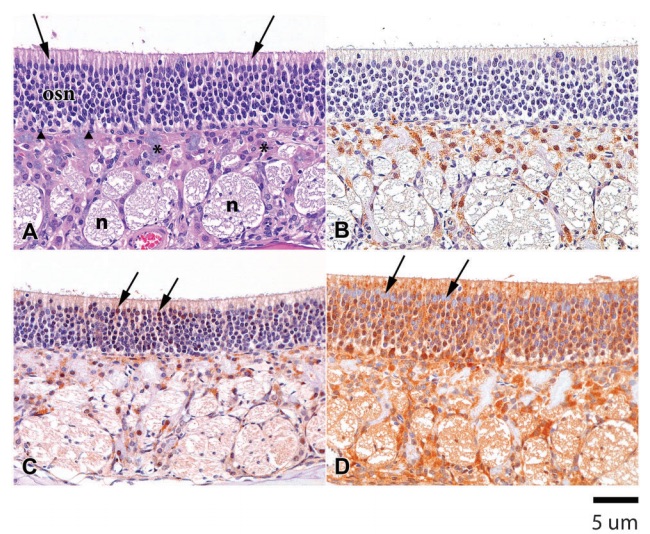
Figure 1 (A) Hematoxylin & eosin (H&E). Rat 1M. Ciliated respiratory epithelium lining the septum in the anterior nasal cavity of a Sprague-Dawley control rat. The respiratory epithelium consists of tall columnar goblet cells (arrows) and basal cells (filled arrowheads). Submucosal glands (open arrowhead) and their glandular ducts (asterisks) are adjacent to the underlying cartilage. (B) CK2a. Rat 1M. Minimal immunopositivity is present in occasional basal cells (filled arrowhead) of the ciliated respiratory epithelium and in apical cytoplasm of a submucosal gland duct (asterisk). (C) P27Kip1. Rat 2M. Moderate immunostaining is present in glandular ducts in the submucosa, especially prominent in the apical cytoplasm (asterisks). Mild punctate immunopositivity is present in the submucosal glands (open arrowhead) and in basal cells of the luminal epithelium (filled arrowheads). (D) HSP90. Rat 2M. Marked HSP90 immunostaining is present in basal cells (filled arrowheads), with mild immunopositivity in glandular ducts (asterisk) and minimal punctate positivity in submucosal glands.
Materials and Methods
Animal exposure
The study protocol was approved by the Institutional Animal Care and Use Committee at the Illinois Institute of Technology Research Institute. Male and female Fischer rats, numbering 344, at 5 weeks of age were exposed to filtered air to serve as controls for a smoke inhalation study. Nose-only exposures were for 3 hours/day for 5 consecutive days. Scheduled necropsies were conducted on study day 5, immediately after final exposure.
Tissue processing/histopathological evaluations
Heads were collected from all animals after the mandibles and skin were removed. Noses from five control rats of each sex were infused with and immersed in neutral buffered formalin (NBF) for 24 hours and then placed in 70% ethanol. Fixed tissue in 70% ethanol was archived for several years. Since tissues from three heads were dessicated based on visual examination, the study was limited to the seven archival samples (four males and three females) that were well preserved without evidence of dehydration. Noses from two males were decalcified in 14% ethylenediaminetetraacetic acid (EDTA) for 18 days. All other noses were decalcified with ImmunoCal (Decal Chemical Corp., Tallman, NY, USA) for 6 days. Following decalcification, tissues were rinsed in tap water, cross-sectioned at four levels, and processed for routine paraffin embedding. Four micron serial sections were mounted on poly-L-lysine glass slides (Mercedes Medical Starfrost Adhesive Slides, Sarasota, FL, USA) for hematoxylin & eosin (H&E) and IHC-staining.
Immunohistochemistry
The IHC staining for CK2a, p27Kip1, and HSP90 involved use of an automated stainer (DAKO Autostainer, Carpinteria, CA, USA) to provide sequential exposure of deparaffinized slides to 3% hydrogen peroxide (Fisher Scientific, Pittsburgh, PA, USA), avidin/biotin (Vector Laboratories, Burlingame, CA, USA), and serum-free protein (DAKO, Carpinteria, CA, USA) blocks followed by application of primary antibody. Sources for primary antibodies were as follows: CK2a (Anti-Casein Kinase 2a, clone 1AD9, Millipore, Temecula, CA, USA), p27Kip1 (C-19, Santa Cruz Biotechnology, Inc, Dallas, TX, USA), and HSP90 (StressMarq, Victoria, BC, Canada). The incubation time and dilution were based on assay optimization studies using selected preliminary sections. Following application of secondary antibody, the ABC label complex (Vector Laboratories, Burlingame, CA, USA) was applied as per kit instructions with DAB (Biocare Medical, Concord, CA, USA) as the chromogen. Stained slides were counterstained with hematoxylin.
CK2a immunostaining
For CK2a staining, the positive tissue type control used was rat intestine. The negative isotype control was purified mouse IgG, with a matching immunoglobulin concentration to that of the primary antibody. Initial studies were performed without antigen retrieval and with primary antibody dilutions of 1:100, 1:200, and 1:500 for an incubation time of 60 minutes. The resultant staining was weak. A second trial using 1:100 and 1:500 dilution after a heat retrieval step in citrate buffer, pH 6, for 45 minutes at 70uC provided too much background staining. In a final trial, using a 1:100 (10 μg/ml) primary antibody dilution without antigen retrieval, slides were incubated for 90 and 120 minutes.
P27Kip1 immunostaining
The positive control for p27Kip1 was rat spleen. Trisbuffered saline served as the negative control. Initial dilutions of primary antibody used were 1:25, 1:50, and 1:100 for 60 minutes incubation time without antigen retrieval. Additional slides were treated with a 1:50 primary dilution after heat retrieval in citrate buffer, pH 6, for 60 minutes at 70°C. Staining was very strong in the 1:25 dilution and the 1:50 with heat-induced epitope retrieval, moderate for the 1:50 without antigen retrieval, and weak for the 1:100 dilution. The final successful trial used a 1:80 (2.5 μg/ ml) primary dilution for 60 minutes without antigen retrieval.
HSP90
For Hsp90 staining, the positive control was rat heart. The negative isotype control was purified mouse immunoglobulin G (IgG). Initial dilutions of primary antibody were 1:40, 1:100, and 1:200 for 60 minutes incubation time without antigen retrieval. The staining was too strong across all three dilutions. In an attempt for better IHC staining, the primary antibody was diluted to 1:400, 1:800, 1:1000, and 1:1200. Also, the secondary antibody was diluted from the initial 1:300 dilution to 1:400. Staining was too dark in the 1:400 and 1:800 dilutions of primary antibody, but began to improve with the final selected 1:1200 (0.83 μg/ml) dilution.
Specific staining details are provided in supplementary material (Supplementary Materials 1–3 http://dx.doi.org/10.1179/2046023613Y.0000000027.S1–S3).
IHC evaluation
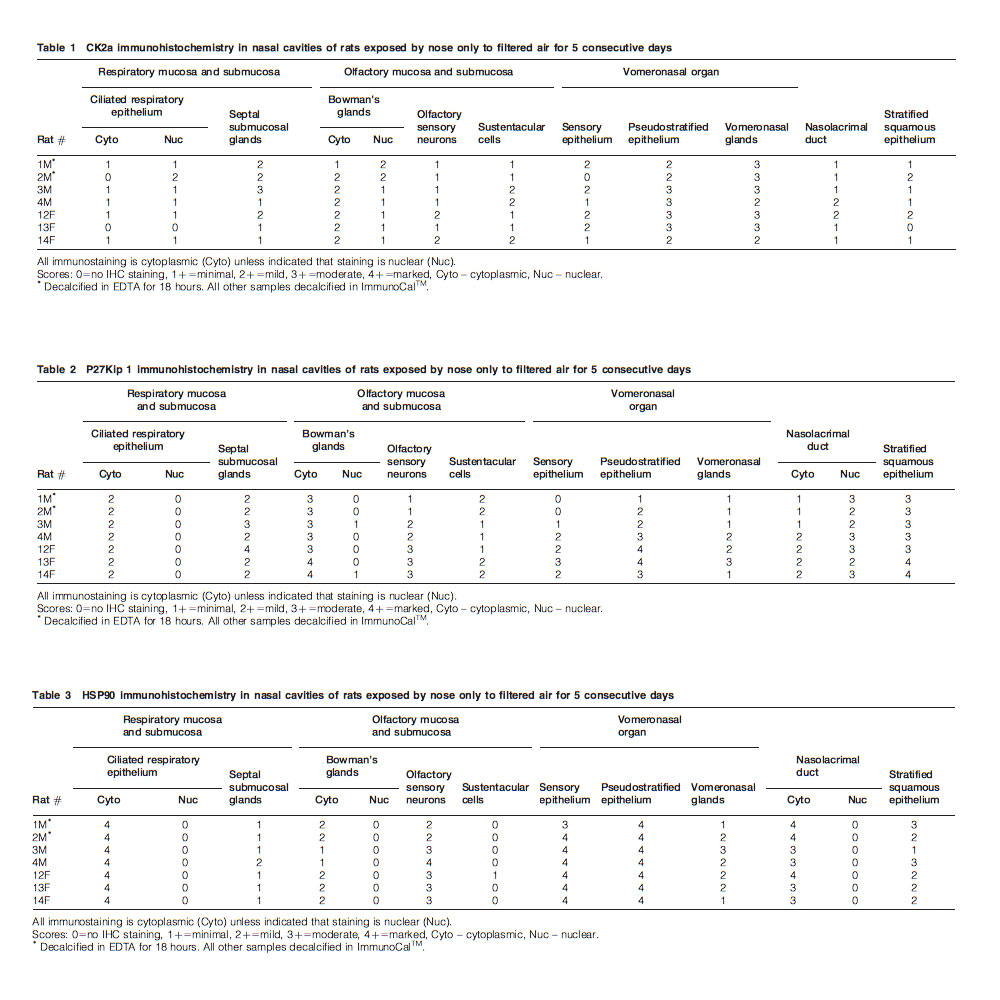
Scoring of IHC was based on the extent and intensity of staining in the various major tissues lining the nasal cavity. Subjective scores were as follows: 0 – no IHC staining present; 1 – minimal IHC positivity; 2 – mild IHC positivity; 3 – moderate IHC positivity; and 4 – marked IHC positivity.
Results
Hematoxylin & eosin staining
There was good representation of the major tissue components in H&E-stained sections from the four cross-sectional levels of the nasal cavity. Tissues included were: stratified squamous epithelium, vomeronasal organ, ciliated and non-ciliated respiratory epithelium, submucosal glands, nasolacrimal duct, and olfactory neuroepithelium with associated Bowman’s glands. Although not evaluated by IHC, the integrity and morphological features of nasal turbinate bones and rodent incisor and molar teeth were indicative of adequate decalcification of the rat noses. For purposes of demonstration, photomicrographs of respiratory epithelium of the nasal septum (Fig. 1A), structures of the vomeronasal organ (Fig. 2A), nasolacrimal duct (Fig. 3A), and olfactory neuroepithelium (Fig. 4A) are provided.
Immunostaining
Good specific immunostaining for CK2a, without objectionable background staining, was obtained with the 120-minute incubation time at 1:100 (10 μg/ml) dilution (Figs. 1B, 2B, 3B, and 4B). No background staining occurred in the negative isotype control. IHC scores for different nasal tissue components are presented in Table 1.
For p27Kip1, good differentiation of cellular components was achieved by using a 1:80 (2.5 μg/ml) primary dilution for 60 minutes (Figs. 1C, 2C, 3C, and 4C). IHC scores for the different nasal tissue components are presented in Table 2.
The 1:1200 (0.83 μg/ml) primary dilution presented strong immunostaining for Hsp90 without excess background (Figs. 1D, 2D, 3D, and 4D). No specific staining was apparent in the negative control. IHC scores for the different nasal components are presented in Table 3.
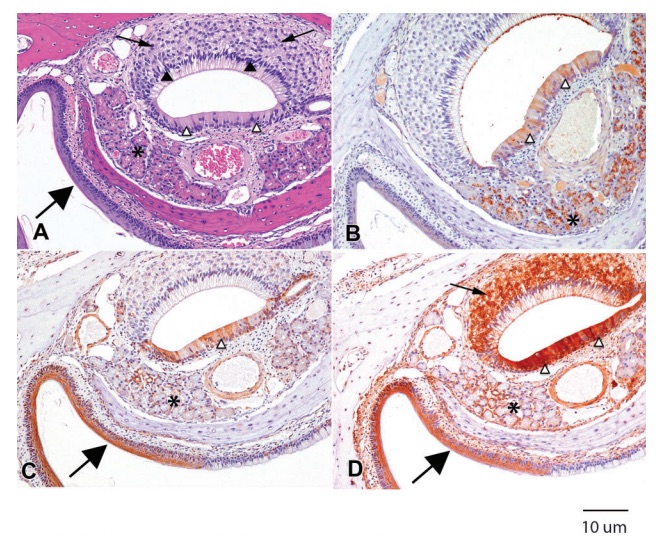
Figure 2 (A) H&E. Rat 1M. Microanatomical features of the vomeronasal organ include bipolar neurons of the sensory epithelium (arrows), supporting cells (filled arrowheads) of the sensory epithelium, pseudostratified epithelium (open arrowheads), and glands (asterisk). The nares are lined by stratified squamous epithelium (large arrow). (B) CK2a. Rat 1M. Mild to moderate immunopositivity is present in the pseudostratified epithelium (open arrowheads) and moderate punctate immunostaining is present in the glands (asterisk). There is a thin line of immunopositivity on the surface of the sensory epithelium. (C) P27Kip1. Rat 2M. There is moderate immunopositivity of the pseudostratified epithelium (open arrowhead) and minimal immunopositivity of the glands (asterisk). The stratified squamous epithelium (large arrow) is strongly positive for p27Kip1. (D) HSP90. Rat 1M. Marked immunostaining is present in the bipolar neurons of the sensory epithelium (arrow), the pseudostratified epithelium (open arrowheads), and the stratified squamous epithelium of the nares (large arrow), with moderate immunopositivity of the glands (asterisk).
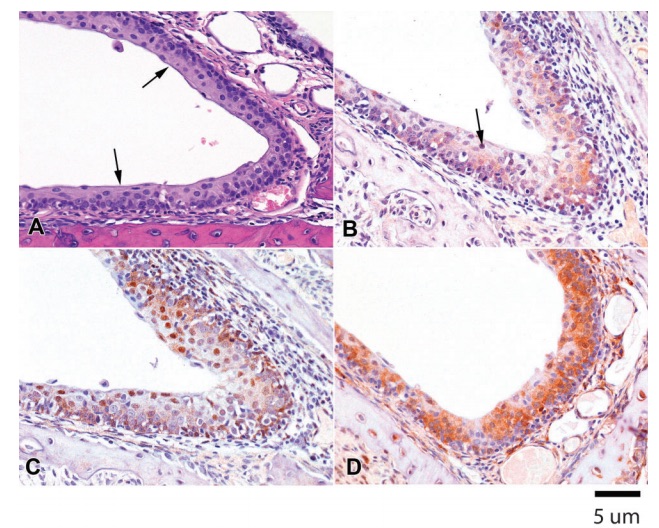
Figure 3 (A) H&E. Rat 1M. Non-keratinized multilayered squamous epithelium (arrows) lines the nasolacrimal duct that drains into the nasal cavity. (B) CK2a. Rat 1M. Minimal to mild cytoplasmic immunostaining is present in the multilayered epithelium of the nasolacrimal duct, with occasional nuclear staining (arrows). (C) P27Kip1. Rat 1M. Mild cytoplasmic and moderate nuclear immunopositivity is present in the nasolacrimal duct epithelium. (D) HSP90. Rat 1M. There is marked cytoplasmic immunostaining for HSP90 in the nasolacrimal duct epithelium.
Figure 4 (A) H&E. Rat 1M. The olfactory epithelium in the posterior nasal cavity of a Sprague-Dawley rat consists of sustentacular cells (arrows), olfactory sensory neurons (osn), basal cells (filled arrowheads), Bowman’s glands (asterisks), and nerve bundles (n) in the lamina propria. (B) CK2a. Rat 4M. Mild immunopositivity is present in the cytoplasm of Bowman’s glands. There is minimal positive staining in the olfactory sensory neurons and sustentacular cells. (C) P27Kip1. Rat 1M. There is moderate immunopositivity for p27Kip1 in the cytoplasm and nuclei of Bowman’s glands as well as a positive response in some of the sustentacular cell nuclei (arrows) and some of the olfactory sensory neurons in the mucosa. There is light staining of the nerve bundles. (D) HSP90. Rat 2M. The olfactory mucosa and submucosa are strongly immunopositive for HSP90, with the exception of the sustentacular cell nuclei (arrows). The staining reaction in Bowman’s gland is limited to the cytoplasm, while the staining reaction in the olfactory sensory neurons is both cytoplasmic and nuclear. There is mild to moderate staining of the nerve bundles.
Discussion
Based on evaluation of the H&E-stained sections, all components of the nasal cavities of the seven rats were within normal limits. As is typical for sections of rodent noses, exact tissue comparison between animals for each sampled level is not perfect due to normal variation in tissue trimming following decalcification. For purposes of illustrating results, photomicrographs (Figs. 1–4) were obtained from the same rat whenever possible. All photomicrographs are from male rats.
Acceptable IHC results on delicate soft tissues were successfully obtained on these archival rodent tissues following decalcification of the surrounding bony structures of the nose. Tissues had been immersion-fixed in NBF for 24 hours, then transferred to and stored in 70% ethanol prior to decalcification and staining. Each of the three IHC markers, Ck2a, p27Kip1, and HSP90, required independent optimization of staining parameters to obtain good specific immunopositivity in the variety of epithelial soft tissues in the nose while ensuring negligible background staining. Antigen retrieval was not necessary for successful immunostaining, although it could be argued that exposure to the decalcification process may represent a form of antigen retrieval. It was noted that staining reactions within cartilage and decalcified bone were variable within and between tissue sections, even on the same slide. This may be a reflection of differential degrees of decalcification of hard tissue or, alternatively, may represent a nonspecific reaction in the biochemical composition of cartilage and decalcified bone. Semi-quantitative evaluation of staining showed similar results between the two decalcification procedures, using either EDTA or ImmunoCal.
While optimizing the Ck2a, p27Kip1, and HSP90, the goal was to obtain a balanced reaction of good immunostaining between the different cellular components of the nasal cavity. The strongest staining was obtained for HSP90, consistent with its high abundance in eukaryotic tissues. While staining optimization for HSP90 yielded acceptable results for all tissue components in the noses of the rats, additional optimization for vomeronasal organ (Fig. 2D) and olfactory epithelium (Fig. 4D) might have improved staining for these two tissue components.
A major challenge during staining was maintaining tissue adherence to the slides during tissue incubation; bony tissue tended to lift from the slide during processing, necessitating cutting additional sections for repeat staining. During early stain optimization, a more gentle method of antigen retrieval was chosen without high pressure or boiling solutions in an effort to reduce this problem. Ultimately, antigen retrieval was not needed for the favorably balanced IHC staining among the different cellular components in the rat nose. Sectioning nasal turbinate blocks at 4 microns, allowing the slides to air dry several days at room temperature prior to staining, and avoiding antigen retrieval steps when possible mitigated loss of tissue.
Conclusion
Antigen retrieval, although necessary for many antibodies, can introduce new challenges to the IHC process. This study using CK2a, p27Kip1, and HSP90 staining showed discrete staining with each antibody in different cell types in the rat nose. The ability to effectively stain for these proteins should aid in predicting the effects of potential toxins on cell proliferation, senescence, differentiation, apoptosis, stress response, and protein folding in specific nasal cell types.
Acknowledgements
Material presented in this paper is from a study funded by Lorillard, Inc., Greensboro, NC, USA.
References
Buchwalow I, Bocker W. Immunohistochemistry: basics and methods. Berlin: Springer-Verlag; 2010.
Zou J, Luo H, Zeng Q, Dong Z, Wu D, Liu L. Protein kinase CK2alpha is overexpressed in colorectal cancer and modulates cell proliferation and invasion via regulating EMT-related genes. J Transl Med. 2011;9:97.
Lin KY, Tai C, Hsu JC, Li CF, Fang CL, Lai HC, et al. Overexpression of nuclear protein kinase CK2 alpha catalytic subunit (CK2alpha) as a poor prognosticator in human colorectal cancer. PLoS One. 2011;6:e17193.
Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC. Protein kinase CK2 in mammary gland tumorigenesis. Oncogene. 2001;20:3247–57.
Rodier G, Montagnoli A, Di Marcotullio L, Coulombe P, Draetta GF, Pagano M, et al. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 2001;20:6672–82.
Esposito V, Baldi A, De Luca A, Groger AM, Loda M, Giordano GG, et al. Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res. 1997;57:3381–5.
Dean DO, Tytell M. Hsp25 and -90 immunoreactivity in the normal rat eye. Invest Ophthalmol Vis Sci. 2001;42:3031–40.
Tomasello G, Sciume´ C, Rappa F, Rodolico V, Zerilli M, Martorana A, et al. Hsp 10, Hsp70, and Hsp90 immunohistochemical levels change in ulcerative colitis after therapy. Eur J Histochem. 2011;55:e38.
Young JT. Histopathologic examination of the rat nasal cavity. Fundam Appl Toxicol. 1981;1:309–12.