Historically, there has been confusion relating to the diagnostic nomenclature for individual cell death. Toxicologic pathologists have generally used the terms ‘‘single cell necrosis’’ and ‘‘apoptosis’’ interchangeably. Increased research on the mechanisms of cell death in recent years has led to the understanding that apoptosis and necrosis involve different cellular pathways and that these differences can have important implications when considering overall mechanisms of toxicity, and, for these reasons, the separate terms of apoptosis and necrosis should be used whenever differentiation is possible. However, it is also recognized that differentiation of the precise pathway of cell death may not be important, necessary, or possible in routine toxicity studies and so a more general term to indicate cell death is warranted in these situations. Morphological distinction between these two forms of cell death can sometimes be straightforward but can also be challenging. This article provides a brief discussion of the cellular mechanisms and morphological features of apoptosis and necrosis as well as guidance on when the pathologist should use these terms. It provides recommended nomenclature along with diagnostic criteria (in hematoxylin and eosin
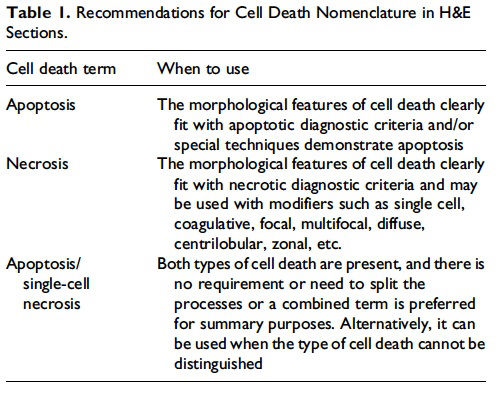
- Use necrosis and apoptosis as separate diagnostic terms.
- Use modifiers to denote the distribution of necrosis (e.g., necrosis, single cell; necrosis, focal; necrosis, diffuse; etc.).
- Use the combined term apoptosis/single cell necrosis when (a) There is no requirement or need to split the processes, or (b) When the nature of cell death cannot be determined with certainty, or (c) When both processes are present together.
- The diagnosis should be based primarily on the morphological features in H&E-stained sections. When needed, additional, special techniques to identify and characterize apoptosis can also be used.
Introduction
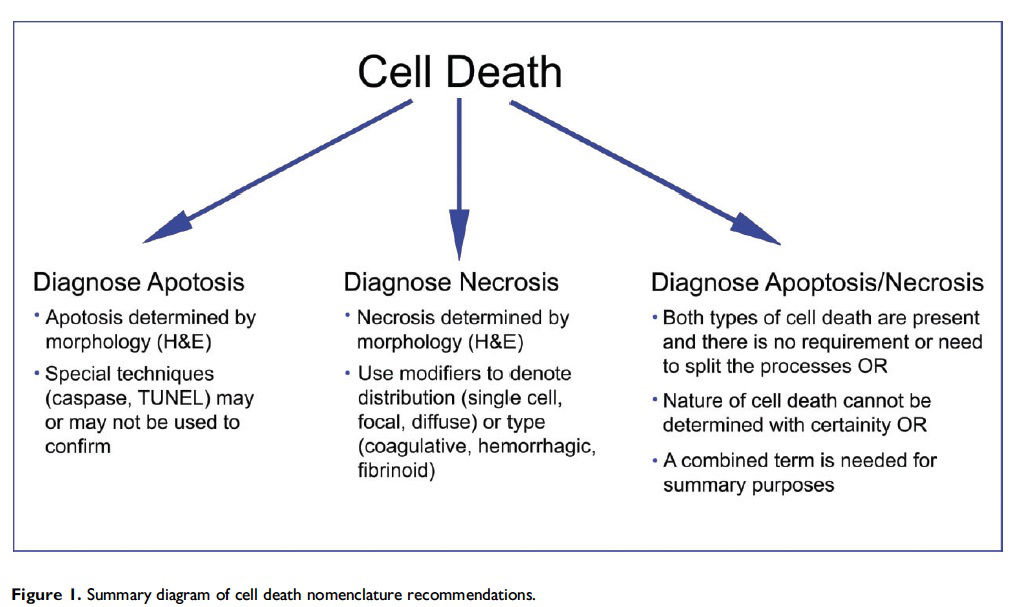
This guidance is for the toxicologic pathologist to use for the routine evaluation of H&E-stained histological sections to distinguish apoptosis and necrosis. This document will serve as current guidance for the International Harmonization of Nomenclature and Diagnostic Criteria (INHAND) Organ Working Groups and the toxicologic pathology community at large. Any previously published INHAND documents that do not align with this guidance will be revised. Importantly, apoptosis and single cell necrosis are not synonyms although previous guidance has indicated otherwise and toxicologic pathologists have often used them interchangeably. We recognize that not all situations demand the same degree of precision with regard to distinguishing apoptotic from necrotic cell death, so the combined term apoptosis/single cell necrosis is available. This term can be used when the distinction between the different types of cell death is not desired or when it cannot be determined by either H&E or other stains that were employed. Recommendations for the use of the terms apoptosis and necrosis as well as the combined term apoptosis/single cell necrosis are discussed in this document and summarized in Figure 1.
Previous guidance on cell death nomenclature was provided in 1999 with the publication of ‘‘The Nomenclature of Cell Death: Recommendations of an ad hoc Committee of the Society of Toxicologic Pathologists’’ by Levin et al. (1999). This Committee recommended that the term ‘‘necrosis’’ be used to describe any morphological findings of cell death in histological sections, regardless of the pathway by which the cells died. Furthermore, ‘‘apoptotic,’’ ‘‘oncotic,’’ or ‘‘mixed apoptotic and necrotic’’ could be used as modifiers to specify the predominant cell death pathway, if appropriate. The overarching goal was that ‘‘pathologists use terms that accurately and concisely convey the level of information appropriate to the study’s needs.’’
However, these terms were not widely accepted in the toxicologic pathology community, and this may have been due to the intense focus of apoptosis research within the scientific community and a global understanding that these two forms of cell death occurred by different mechanisms. To address the issue of cell death nomenclature in the scientific community, an international group of scientists first published ‘‘Cell Death and Differentiation’’ in 2005 and then provided the updated ‘‘Classification of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2009’’ 4 years later (Kroemer et al. 2005; Kroemer et al. 2009). They proposed unified criteria for the definition of cell death and its different morphologies. Definitions were provided for apoptosis, necrosis, autophagy and cornification, and tentative definitions of atypical cell death modalities (mitotic catastrophe, anoikis, Wallerian degeneration, paraptosis, pyroptosis, and pyronecrosis) were discussed. They recommended that specific biochemical analyses (i.e., DNA ladders) should not be used as exclusive tests for apoptosis. Likewise, proteolytically active caspases or cleavage products of their substrates are not sufficient to define apoptosis. However, it was stated that these tests, along with other features, may be helpful in the diagnosis of apoptosis. Part of the conclusions stated that there are 3 distinct routes of cellular catabolism that can be defined by morphological criteria: autophagy, necrosis, and apoptosis. The use of terms such as necroapoptosis and aponecrosis to describe cell death, where apoptosis and necrosis occur simultaneously, was discouraged and instead they recommended using necrosis and apoptosis as separate terms.
There have been numerous advances in the field of cell death since the Levin et al. (1999) publication. Although the mechanisms and morphologies of apoptosis and necrosis differ, there is evidence that they share a similar biochemical network, described as the ‘‘apoptosis–necrosis continuum’’ (Zeiss 2003). As an example, a decrease in the availability of caspases and intracellular adenosine triphosphate (ATP) will convert an ongoing apoptotic process into a necrotic process (Denecker et al. 2001; Leist et al. 1997). Although the two processes can overlap due to available substrates and energy, there is and has been clear evidence that necrosis and apoptosis occur via different pathways.
This article provides guidance on the identification and diagnosis of apoptosis and necrosis. This guidance document also includes discussion of the cellular mechanisms of apoptosis and necrosis, when and where apoptosis occurs, descriptions of morphological features of apoptosis and necrosis in H&E-stained tissue sections, additional techniques that can be used to confirm apoptosis, and some organ-specific examples of when distinguishing between apoptosis and single cell necrosis may be challenging.
There are other forms of cell death that have been described and discussed in the literature such as autophagy, necroptosis, eryptosis, anoikis, and pyroptosis (Bergsbaken, Fink, and Cookson 2009; S. A. Elmore 2015; Gilmore 2005; Lang, Lang, and Foller 2012; Linkermann and Green 2014; Newton 2015; Ryter, Mizumura, and Choi 2014). These other forms of cell death are beyond the scope of this article; therefore, they will not be discussed here.
Recommendations for Cell Death Nomenclature
Apoptosis
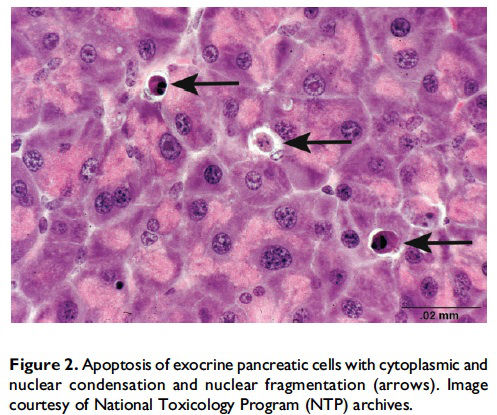
Review of cellular mechanisms of apoptosis. Apoptosis, also described as ‘‘programmed cell death,’’ was first described by Kerr, Wyllie, and Currie (1972) and is a process of cell death that is mediated by a genetically controlled, energyrequiring program. The 3 main pathways of apoptosis are (1) targeting mitochondria functionality (mitochondrial or intrinsic pathway), (2) direct transduction of the signal via adaptor proteins (death receptor or extrinsic pathway), and (3) the perforin/granzyme pathway (S. Elmore 2007). One of the key features of apoptosis is the cleavage of cytoskeletal proteins by aspartate-specific proteases, which results in the collapse of subcellular components. This can be seen histologically as cytoplasmic and nuclear condensation and nuclear fragmentation (Figure 2). Importantly, for all 3 pathways, plasma membrane integrity persists until late in the process. This prevents release of cellular components into the cytosol and thus the lack of inflammation. Eventually, apoptotic cells and fragments (apoptotic bodies) will externalize phosphatidylserine on the cell membrane, which results in recruitment and engulfment by macrophages (tingible body macrophages).
The mechanisms of apoptosis are highly complex and sophisticated, involving an energy-dependent cascade of molecular events (S. Elmore 2007). The intrinsic pathway is characterized by increased permeability of the mitochondria, release of cytochrome c, formation of a multiprotein complex called the ‘‘apoptosome,’’ and initiation of the caspase cascade through caspase 9. The extrinsic pathway involves proapoptotic ligands (i.e., FasL/FasR, tumor necrosis factor [TNF]-α/ TNFR1, etc.), binding with transmembrane death receptors (members of TNF receptor gene superfamily), transduction of intracellular signals, binding of adaptor proteins, deathinducing signaling complex formation, and then initiation of the caspase cascade through caspase 8. The perforin/granzyme pathway is involved in T cell-mediated cytotoxicity and is characterized by secretion of the transmembrane poreforming molecule perforin, exophytic release of cytoplasmic granules containing serine proteases (granzymes) through the pore and into the target cell, and then either initiation of the caspase cascade through caspase 10, direct initiation of caspase 3, or a caspase independent DNA cleavage via a SET complex (nucleosome assembly protein SET, apurinic/apyrimidinic endonuclease 1 [Ape1], protein phosphatase 32 [pp32], High mobility group protein 2 [HMG2]). Factors that determine which apoptotic pathway is activated include the stage of the cell cycle, the type and magnitude of stimuli, and, for immune cells, the stage of cellular activation. Different apoptotic pathways may occur concomitantly. Unlike necrosis, apoptosis can be, under certain circumstances, reversible. One example is the reversibility of p53-induced apoptosis (Geske et al. 2001). The tumor suppressor p53 is activated by a variety of cellular insults such as damaged DNA, nucleotide depletion, hypoxia, and heat shock. Geske and colleagues have shown that p53-induced apoptotic cells can be rescued from the early stages of the apoptotic process. DNA repair was shown to be activated early in p53-induced apoptosis and could modulate the cell death process if the apoptotic stimulus was removed.
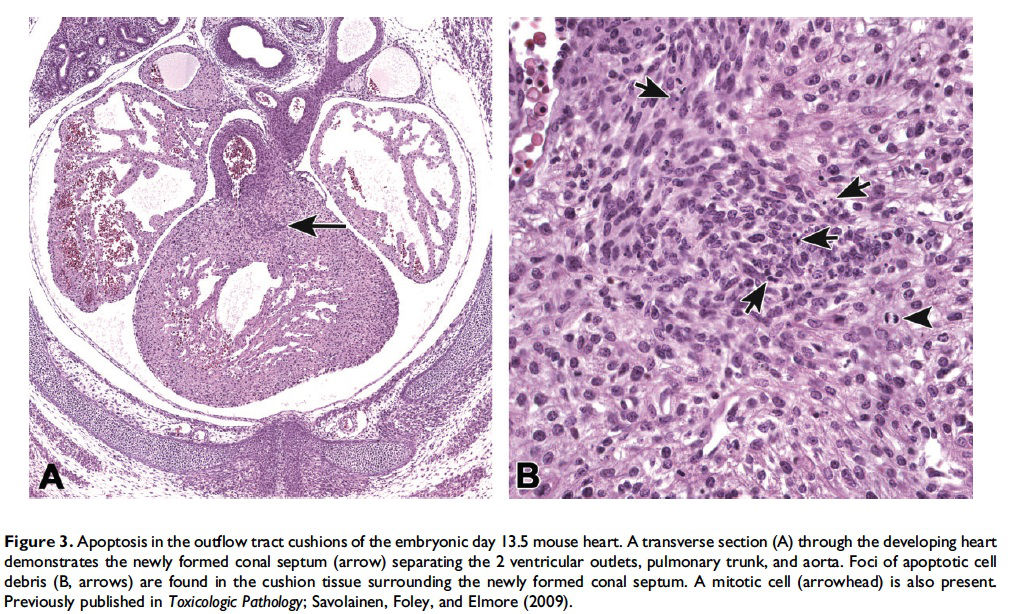
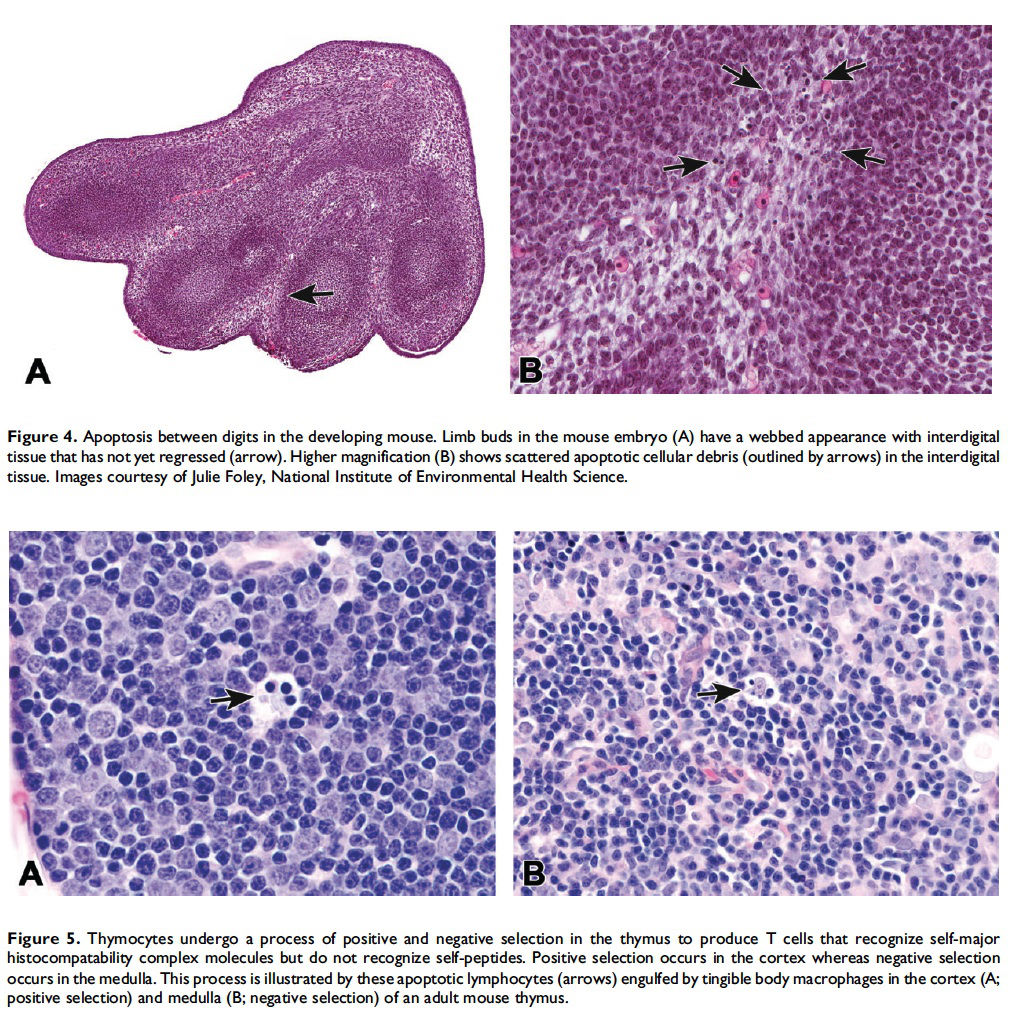
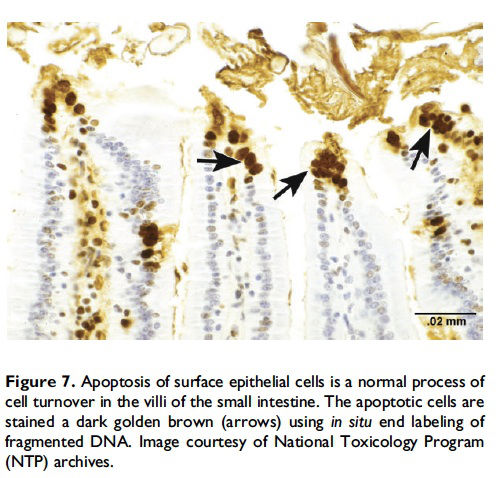
When and where apoptosis occurs. In multicellular organisms, apoptosis can occur in both developing and adult tissues, during normal tissue homeostasis, and as a result of different types of cellular insults (Dexter et al. 1995; Meier, Finch, and Evan 2000). It is an important mechanism for the removal of senescent, superfluous, infected, transformed, or damaged cells. During development, apoptosis is a normal and necessary component of organ/tissue development. It occurs in developing tissues and organs at specific times and regions, and is involved in the developmental remodeling of tissues by targeting transient cells, allowing for further tissue differentiation. As an example, one of the primary sites of apoptosis in the normal developing heart are the atrioventricular cushions (Figure 3; Savolainen, Foley, and Elmore 2009). In the developing nervous system, up to half or more of the nerve cells normally die soon after they are formed so that the number of nerve cells matches the number of target cells that require innervation (Raff et al. 1993). Another classic example of apoptosis during development involves the hand or paw; individual digits separate only as the cells between them undergo apoptosis (Figure 4; Chimal-Monroy et al. 2011). Apoptosis is also a normal and necessary form of cell death in adult tissues. In the thymus, apoptosis is involved in both positive and negative selection of developing thymocytes, ensuring that T cells that recognize self-antigen are eliminated before they leave the thymus, thus avoiding autoimmunity (Figure 5; Giovannetti et al. 2008). Similarly, apoptosis is necessary during B-cell development in the bone marrow (Lu and Osmond 2000). In activated lymph nodes, apoptosis occurs in germinal centers as a component of antibody-mediated immunity (Figure 6; Rizzo et al. 1998). There are many examples where apoptosis helps regulate cell number in adult tissues. In the gastrointestinal tract, apoptosis most likely accounts for the bulk of cell loss (Figure 7; Hall et al. 1994). Normal cell death for adult tissues exactly balances cell division. Otherwise, the tissues would grow or shrink. Inhibition of apoptosis can result in a variety of conditions including cancer, autoimmune disease, inflammatory disease, and viral infection. On the other hand, increased or excessive apoptosis can lead to conditions such as neurodegenerative diseases, hematologic diseases, and tissue damage (Fuchs and Steller 2011). Physiologically or toxicologically induced reductions in endocrine hormones often cause apoptosis in hormonally dependent tissues—for example, ovary, uterus, epididymis (Figure 8), seminal vesicles, prostate, and pancreas (Figure 2).
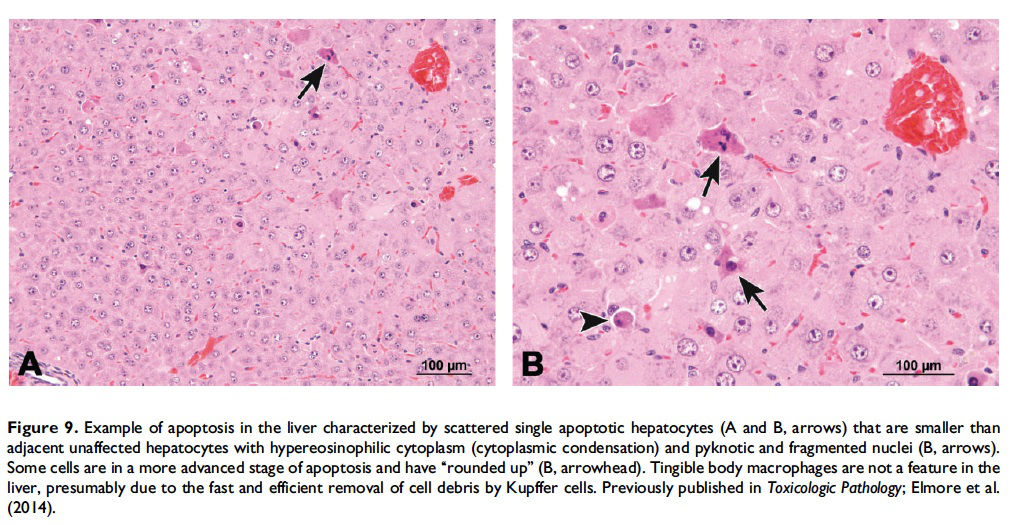
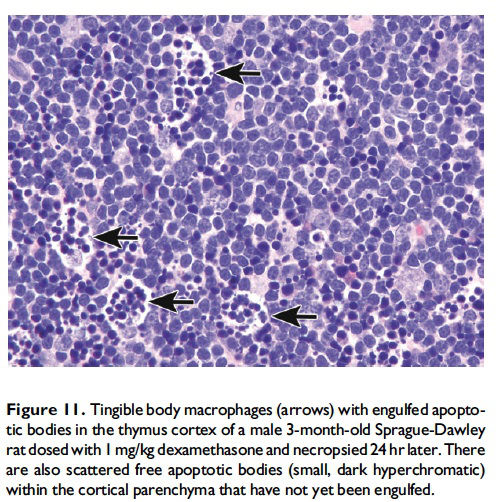
Description of morphological features of apoptosis in H&E-stained tissue sections. In general, apoptotic cells occur as single, noncontiguous cells or small clusters of cells alone or scattered within a tissue section (Figure 9 and Table 1; S. A. Elmore et al. 2014). However, experimental induction can result in massive apoptosis of all or most cells (Figure 10). The classic apoptotic cell is smaller than the surrounding cells with a small pyknotic nucleus (due to chromatin condensation) and, depending on the cell type, hypereosinophilic cytoplasm (due to cytoplasmic condensation; Figure 2). As an example, hepatocytes will have more hypereosinophilic cytoplasm than lymphocytes, which generally have a larger nucleus to cytoplasmic ratio. The nucleus may also be fragmented (karyorrhexis). Fragments of apoptotic cells, called ‘‘apoptotic bodies,’’ are formed by the induction of caspase 3-mediated cytoskeletal reorganization and disintegration (S. Elmore 2007; Taylor, Cullen, and Martin 2008). Apoptotic bodies have either a pyknotic nuclear fragment with or without the associated cytoplasm or cytoplasm alone. Tingible body macrophages, which are macrophages containing one or more engulfed cytoplasmic apoptotic bodies, may also be present (Figure 11). It is important to remember that tingible body macrophages may not be seen in all tissues, such as the liver. They can be a normal finding in some tissues, most commonly the thymus cortex and germinal centers of lymph nodes and spleen. They may also be present in the T-cell regions of all lymphoid organs as a result of treatment and in lymphoid neoplasms (‘‘starry-sky’’ effect). Because the cell membrane of an apoptotic cell is not compromised, there is generally no inflammation associated with apoptosis. However, an occasional apoptotic cell may be associated with inflammatory foci (Figure 12). Only an increase in tingible body macrophages may be present at times, with no accompanying free apoptotic cells in the surrounding tissue. This is indicative of a prior increase in apoptosis where all of the apoptotic bodies have been engulfed by macrophages. In this case, only an increase in tingible body macrophages would be diagnosed, and the pathogenesis discussed in the pathology narrative.
Additional techniques that can be used to confirm apoptosis. Sometimes it may be difficult to distinguish apoptosis from necrosis using cellular morphology on H&E-stained slides. If it is important to distinguish which process (apoptosis or necrosis) is occurring, then a variety of special tests may be performed (Table 2; Krysko et al. 2008). However, there is no one ‘‘correct’’ or ‘‘best’’ test for every situation. The choice of additional tests must be determined based on time, cost, skill level, and so on. The choice of additional tests may also vary depending on whether or not the tissue review is part of an initial study review or part of a more comprehensive research project. The most commonly used tests include caspase 3, a key effector in the apoptosis pathway, and the terminal deoxynucleotidyl transferase deoxyuridine triphosphate (dUTP) nick end labeling (TUNEL) assay, which labels and detects blunt ends of double-stranded DNA breaks. Regardless of which additional tests are used, transmission electron microscopy (TEM) remains the gold standard. Although TEM is more time consuming, expensive and requires special fixation, the results are unequivocal.
There are numerous techniques, other than morphological evaluation, that can be used to confirm and/or better define apoptosis, but these should be used with an understanding of the benefits and limitations of each test. A few of the more commonly used assays are discussed in detail below (DNA laddering assay, various caspase assays, and the TUNEL assay). Additional tests include analysis of cell morphology by real-time imaging (time-lapse microscopy); differential interference contrast microscopy (DIC); combination of DIC and epifluorescence microscopy; flow fluorocytometry (FACS) of cell morphology and cell permeability; analysis of surface changes (phosphatidylserine exposure) by FACS; in situ end labeling of fragmented DNA in formalin-fixed tissue (Figure 7); activation of caspases; cleavage of BH3 interactingdomain death agonist [BID] (a proapoptotic member of the Bcell lymphoma 2 family) and release of cytochrome c; analysis of cytokeratin 18 release by ELISA; in vitro phagocytosis assay by FACS; and analysis of micro- and macropinocytosis internalization processes (Krysko et al. 2008; Wijsman et al. 1993). However, analysis of cell morphology by TEM is considered the gold standard in cell death research for confirmation of apoptosis (S. Elmore 2007). Classic features of apoptosis include an electron dense nucleus (marginalization in early phase); nuclear fragmentation; intact cell membrane, disorganized cytoplasmic organelles; and irregular cell contour with blebs at the cell surface (White and Cinti 2004). As apoptosis progresses, the cells lose cell-to-cell adhesion and separate from neighboring cells. Finally, the apoptotic bodies are engulfed by tingible body macrophages, other phagocytizing cells, or surrounding cells.
The historical test for apoptosis is the DNA laddering assay (Zakharyan and Pogosyan 1978). This test depends on cleavage of chromosomal DNA into oligonucleosomal size fragments, which was, for many years, considered the biochemical hallmark of apoptosis. Endogenous endonucleases cleave chromatin DNA into multiples of approximately 180 bp fragments (i.e., 360 bp, 540 bp, etc.) producing a characteristic laddering effect using gel electrophoresis and ethidium bromide staining. Necrosis, on the other hand, would produce a smear. However, the caveat to using this test alone is that apoptosis can occur without oligonucleosomal DNA fragmentation (Iguchi, Hirano, and Ishida 2002; Yamaguchi et al. 2004; Yuste et al. 2001).
There are a variety of caspase assays including those that target initiator caspases (8 extrinsic pathway, 9 intrinsic pathway, and 10 perforin/granzyme pathway), those that target effector caspases (1, 2, 3, 4, 5, 6, 7, 11, 12, 13, and 14), and a poly caspase kit (Kaufmann et al. 2008). Tests for caspase activity can be performed on formalin-fixed paraffinembedded tissues, frozen tissues, and cultured cells (whole cells or cell lysates). Live cells in microplate wells can be monitored in real time for apoptotic activity. One caveat of the caspase assay is that although there are, at present, 14 known caspases, it is unclear whether all participate in apoptosis (Slee, Adrain, and Martin 1999). Caspase 3 is the most popular assay because it is a member of the apoptosis executioner group and is processed by any of the initiator caspases (8, 9, and 10) or granzyme B. The granzyme A pathway is caspase independent. Because some target different times of apoptosis (early vs. late) and different pathways of apoptosis, discretion should be used when choosing the best assay(s) for any particular study.
The TUNEL assay detects DNA fragmentation by labeling the terminal end of nucleic acids (Gavrieli, Sherman, and Ben-Sasson 1992). The enzyme terminal deoxynucleotidyl transferase catalyzes the addition of biotinylated dUTPs to DNA nicks. However, it will also label any cell that has severe DNA damage and DNA fragmentation. The original assay was very nonspecific, labeling both apoptotic and necrotic cells. However, success of the TUNEL assay relies on the markedly larger number of DNA breaks that occur during apoptosis. This method has reportedly been improved in subsequent years, and reports suggest that it will now more specifically identify cells in the late phase of apoptosis (Loo 2011; http://lifescience. roche.com/wcsstore/RASCatalogAssetStore/Articles/BIO CHEMICA_98_3_p36-41.pdf, accessed 10/1/14).
When the pathologist should use the term apoptosis. The term apoptosis should be used when there is adequate morphological evidence to provide confidence in the diagnosis. That is, there should be cell and nuclear shrinkage and nuclear pyknosis. There may or may not be hypereosinophilic cytoplasm, depending on cell type (Figures 2–6 and 8–10). If the process has progressed, then apoptotic bodies and tingible body macrophages may also be present, depending on the tissue type (Figure 11). Inflammation is generally not a feature of apoptosis. Apoptosis may occur in conjunction with necrosis and when this occurs, both diagnoses should be used. These may be diagnosed as separate terms or the combination term apoptosis/single cell necrosis may be used (see below), depending on the need or requirement of the study.
Necrosis
Review of cellular mechanisms of necrosis. Necrosis of single cells and contiguous cells is irreversible and most often results from acute cellular injury: metabolic failure of the cell that coincides with rapid depletion of ATP. In terms of cellular mechanisms, there are no published studies that indicate that single cell necrosis is mechanistically different from focal or diffuse necrosis where groups of contiguous cells are affected. In contrast to apoptosis, necrosis has not historically been considered to be a genetically controlled process that requires energy. However, despite our historical understanding of necrosis as a passive and ‘‘accidental’’ process that is unregulated and uncontrollable, there is experimental evidence in Caenorhabditis elegans and mammalian cells that indicates that some forms of necrosis are ‘‘programmed’’ or actively mediated by the dying cell. Activation of the kinase domain of the receptor interacting protein 1 (RIP1) and assembly of RIP1/RIP3- containing signaling complex have been shown to trigger ‘‘programmed necrosis’’ in some cells in vitro, a process termed ‘‘necroptosis.’’ The process of necroptosis is complex and is triggered by members of the TNF family, requires inhibition of caspase 8, and assembly of RIPK1–RIPK3 complex IIb, also called the ‘‘necrosome’’ (Cho et al. 2009; Han, Zhong, and Zhang 2011; He et al. 2009; Holler et al. 2000; Kaczmarek, Vandenabeele, and Krysko 2013; Kung, Konstantinidis, and Kitsis 2011; Loo 2011; Proskuryakov, Konoplyannikov, and Gabai 2003; Vandenabeele et al. 2010; D. W. Zhang et al. 2009). This type of cell death has been shown to participate in the pathogenesis of a variety of diseases including ischemic injury, neurodegeneration, and viral infection. However, it is not histologically distinguishable from ‘‘classic’’ necrosis (Vanden Berghe et al. 2010). Therefore, this is a term that can only be used when additional in vitro experimental molecular methods are applied.
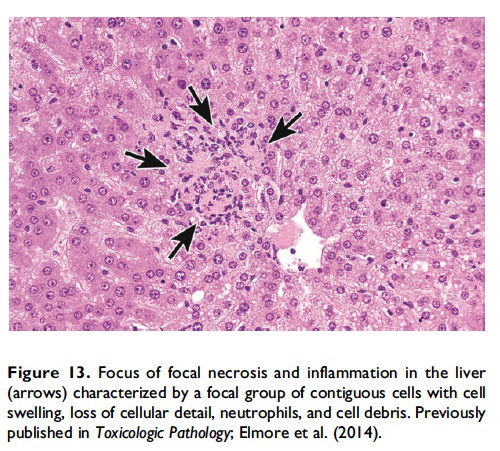
The basic process of necrosis involves loss of cell membrane integrity, which allows for an influx of extracellular ions and fluid, with resultant swelling of the cell and its organelles. Another cellular mechanism of necrosis involves the compromise of organelle membranes, which allows proteolytic enzymes to escape from lysosomes, enter the cytosol, resulting in unregulated digestion of cellular components and destruction of the cell. By electron microscopy, the necrotic cell exhibits some common morphological features such as an increasingly translucent cytoplasm, organelle swelling, dilatation of the nuclear membrane and condensation of the chromatin into small, irregular, circumscribed patches, and increased cell volume (Vandenabeele et al. 2010). This increased cell volume will eventually result in disruption of the plasma membrane with uncontrolled leakage of cellular components (with inflammatory mediators) into the cytosol and interstitial space and subsequent recruitment of inflammatory cells (Figure 13; Shen, Kreisel, and Goldstein 2013).
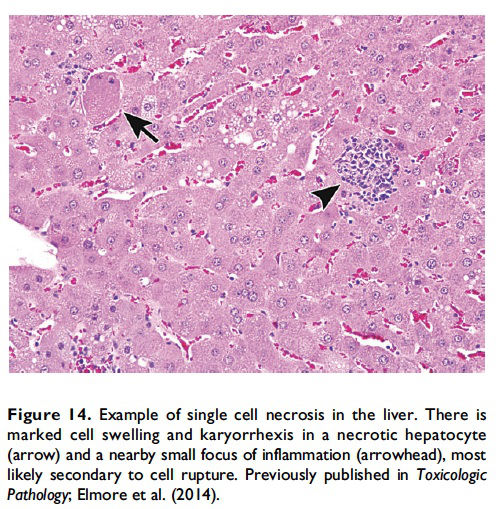
Description of morphological features of necrosis in H&E-stained tissue sections. Necrosis can occur in any tissue and is characterized by loss of cellular detail in a single cell or in contiguous cells and is generally associated with cellular debris and inflammation (Figures 13 and 14; Table 1; Ziegler and Groscurth 2004). The pattern may vary depending on the nature of the insult and the tissue (single cell, focal, multifocal, diffuse, centrilobular, zonal, etc.). In general, ‘‘necrosis, single cell’’ describes single, noncontiguous cells in a tissue that are characterized by cell and nuclear swelling and pale cytoplasm (Figure 14, arrow; Table 1). This is in contrast to the smaller, shrunken hypereosinophilic apoptotic cell (Figure 2). Necrotic cells can also show nuclear dissolution (karyolysis) and/or nuclear fragments (karyorrhexis). Some degree of nuclear condensation (pyknosis) may be present, although less than what occurs with apoptosis. Adjacent cellular debris and inflammation (neutrophils, macrophages, etc.) may also be present if cell membrane leakage or rupture has occurred (Figure 14, arrowhead).
Similarly, focal or diffuse areas of necrosis will contain contiguous cells that have the same characteristics as necrotic single cells. Cell swelling, nuclear swelling, karyolysis, karyorrhexis, nuclear pyknosis, pale eosinophilic cytoplasm, ± cytoplasmic vacuoles may be present in areas of necrosis. Loss of cellular detail with only ghost cells remaining could also be present, which should not be confused with autolysis. If ischemia or infarction has occurred, then coagulative necrosis may be present, which is characterized by preservation of architecture but with pale, eosinophilic cells with no purple, viable nuclei remaining. This unique feature is due to a lack of lysosomal enzymes and thus no proteolysis of damaged cells.
When the pathologist should use the term necrosis. The term necrosis should be used in conjunction with a modifier such as single cell, focal, multifocal, or diffuse to define the distribution. The term necrosis, single cell should be used when there is adequate morphological evidence to provide confidence in the diagnosis. That is, there should be cell and nuclear swelling and somewhat paler eosinophilic cytoplasm than the adjacent cells. There may also be nuclear dissolution (karyolysis) and/or fragmentation (karyorrhexis; Figure 14). Inflammation may or may not be present with necrosis of single cells. When there are contiguous necrotic cells with the characteristic features described above, other common distribution modifiers for necrosis should be used such as ‘‘necrosis, focal’’; ‘‘necrosis, multifocal’’; and ‘‘necrosis, diffuse’’ (Figure 13). Tissue distribution may also be indicated as in ‘‘liver, centrilobular’’ or ‘‘kidney, inner/outer stripe.’’ In addition, necrosis may display certain cytological features that are considered important to capture, such as coagulative, hemorrhagic, fibrinoid, and so on, and these can also be included as descriptive modifiers. Inflammation generally accompanies contiguous cell necrosis, except in the case of coagulative necrosis.
Apoptosis/Single cell Necrosis
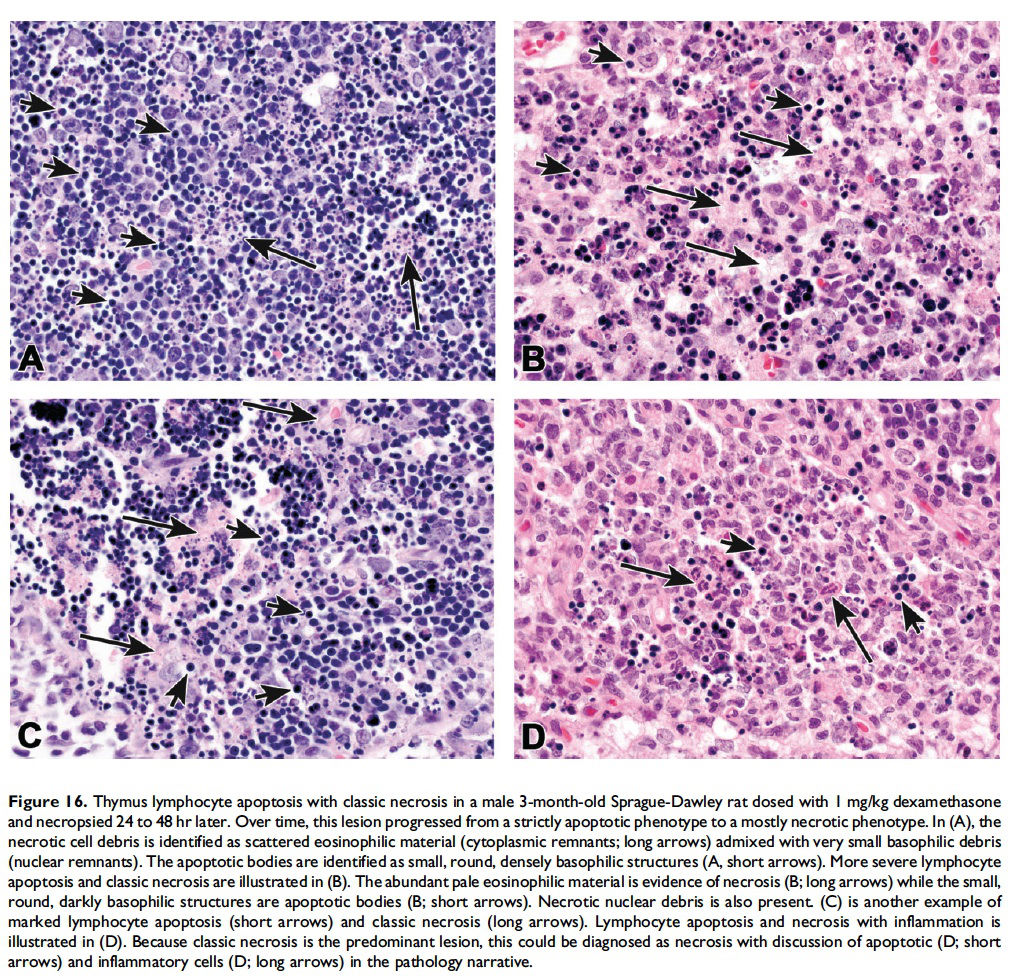
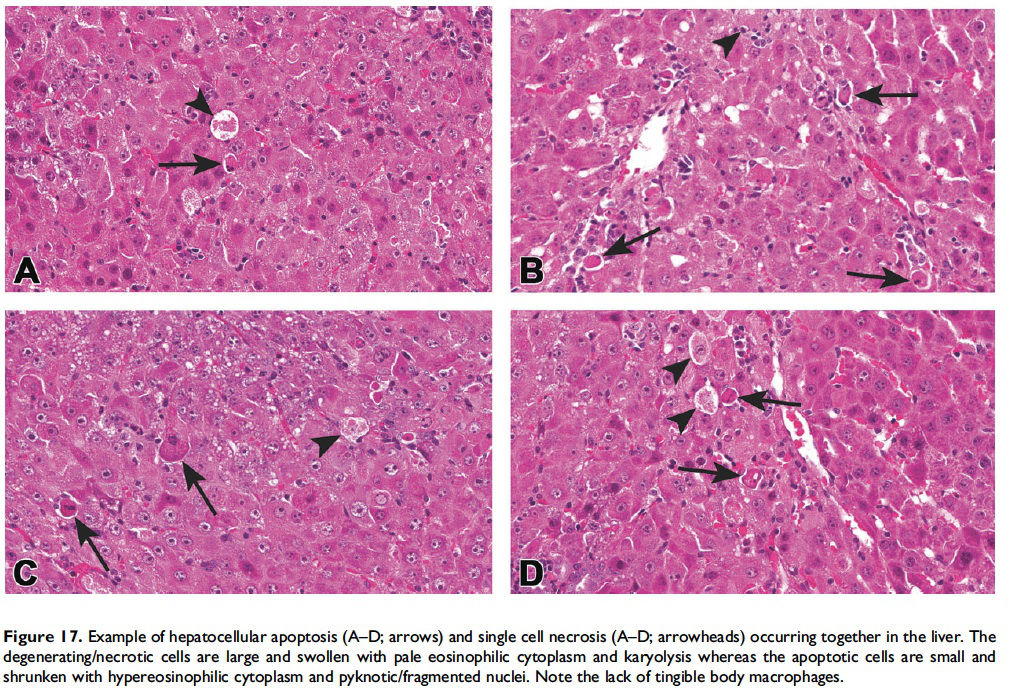
When the pathologist should use the combined term ‘‘apoptosis/ single cell necrosis’’. For the routine evaluation of tissues in regulatory toxicity studies, it may not be necessary or it may be preferable to categorize similar cellular outcomes (e.g., individual cell death) under a single term, rather than subdivide the process. In these situations, apoptosis/single cell necrosis can be used as a combination term to include both processes (Figures 15–17). In addition, the combination term can be used when the pathologist cannot distinguish between the two processes based on the cytological features in H&E-stained sections.
Apoptosis and Classic Necrosis
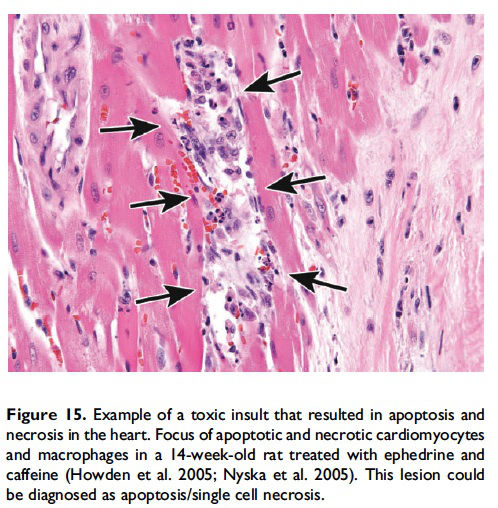
While it is preferable to separately identify and record diagnoses of apoptosis and classic necrosis, this distinction may not be possible when these processes occur together. This may be because one type of cell death histologically obscures the other. Also, necrotic cell debris can have some similarities to apoptotic debris, such as pyknosis and karyorrhexis or vice versa. Apoptosis may predominate, with conversion to a necrotic phenotype or necrosis may predominate with scattered apoptosis. In these cases, it would be appropriate to use both terms or only indicate the predominate type of cell death and discuss the presence of the other type of cell death in the narrative (Figures 15–17; Howden et al. 2005; Nyska et al. 2005). Discussion of the presence of both types of cell death (apoptosis and classic necrosis) may provide the reader with insight into the pathogenesis of the lesion, so a detailed explanation may be provided in the pathology narrative.
Organ-specific Examples
The distinction between apoptosis and single cell necrosis is easier in some tissues than others, and the kidney and brain provide two practical examples of challenges that might be encountered. The renal tubular epithelium is a particularly difficult tissue to distinguish apoptosis from necrosis. There are cases of straightforward epithelial cell necrosis (Figure 18A); however, there are lesions with cells that have historically been diagnosed as single cell necrosis but that have a morphology of classic apoptosis (Figure 18B). Additionally, these two types of cell death frequently occur together. Factors such as nucleotide depletion, electrolyte imbalance, reactive oxygen species, endonucleases, disruption of mitochondrial integrity, and activation of various components of the apoptotic machinery have been implicated in renal cell vulnerability (Padanilam 2003). Two major functions that may determine apoptosis or necrosis in the tubular epithelium are attachment to the basement membrane and oxygen availability (Lieberthal and Levine 1996). A tubular cell undergoing early apoptosis may convert to necrosis if the basement membrane attachment becomes disrupted. As such, necrotic tubular cells may appear shrunken, hypereosinophilic, and desquamate. Regarding oxygen availability, cells with low available oxygen tend to undergo necrosis rather than just apoptosis, while adjacent cells with adequate perfusion follow apoptotic patterns. For these reasons, and probably others, these two forms of cell death often occur together when an agent or condition produces necrosis of a single cell.
The issue of necrosis versus apoptosis in the nervous system is also more complex than it seems at first glance. During development, a considerable amount of neuronal cell death occurs normally, particularly during certain stages, and it is generally accepted that this neuronal cell death occurs via the apoptotic pathway (Dekkers and Barde 2013). However, in adult animals, most pathologists have been taught that the classic ‘‘dead red’’ neuron, which is the most common morphologic manifestation of neuronal death, is a necrotic cell (Figure 19A; Morgan et al. 2004). During the past 10 to 15 years, a substantial amount of research has been done to characterize the mechanism of cell death resulting from various toxicants (e.g., domoic or kainic acid, trimethyltin chloride [TMT], and lead) and pathologic conditions (e.g., seizures, cortical trauma), and it is becoming increasingly apparent that both necrosis and apoptosis can occur in response to the same type of insult, and with similar morphological features with H&E staining (Figure 19B; Ettcheto et al. 2015; Giordano et al. 2008; Glassford et al. 2002; Krajewska et al. 2011; Martin et al. 1998; Wang et al. 2005; X. M. Zhang and Zhu 2011). Morphologically, one may have a sense for which process predominates based on cellular features (Figure 19C), the extent of neuronal loss/death, the amount of tissue damage, and the glial/inflammatory response, but it may be difficult to discern with certainty in most cases without special stains. And, even if special stains were applied, the results may indicate that both are occurring.
Based on these diagnostic difficulties in the kidney and brain, it is recommended that apoptosis or necrosis be used if there is confidence (either through experience or special studies) in the type of cell death present. However, if the pathologist is unsure of the type of cell death, if both occur together, or if there is no need to distinguish the type of cell death, then the combined apoptosis/single cell necrosis term can be used.
Conclusions
Updated guidance for cell death terminology is needed in the field of toxicologic pathology due to continued advances in our understanding of cellular mechanisms of apoptosis and necrosis and the need for international nomenclature harmonization. This document gives guidance on the use of diagnostic criteria for apoptosis and necrosis and is summarized in Figure 1. Importantly, necrosis should not be used as an umbrella term for apoptosis. If single cell necrosis is present, then it should be diagnosed as necrosis with the modifier ‘‘single cell.’’ In situations when there is no need or requirement to specify the type of cell death, there is inadequate morphological evidence to provide confidence in using the separate diagnoses, or there is a need to combine the different forms of cell death for summary purposes, the combination term apoptosis/single cell necrosis should be used. The decision of which diagnostic term to use will depend on the particular organ or tissue, the context of the study, and the discretion of the pathologist.
Acknowledgments
The authors would like to thank Drs. James Morrison, Robert Garman, Roland Auer, John Seely, and Kendall Frazier for helpful discussions for the section on diagnostic dilemmas in the brain and kidney.
Authors’ Contribution
Authors contributed to conception or design (SE, DD, JH, TH, RH, RM, TN, JR, SR, TR, HS, JV, CW, and DC); data acquisition, analysis, or interpretation (SE, DD, JH, TH, RH, RM, TN, JR, SR, TR, HS, JV, CW, and DC); drafting the manuscript (SE); and critically revising the manuscript (SE, DD, JH, TH, RH, RM, TN, JR, SR, TR, HS, JV, CW, and DC). All authors gave final approval and agreed to be accountable for all aspects of work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported [in part] by the Intramural Research Program of the National Institutes of Health (NIH) and National Institute of Environmental Health Sciences (NIEHS).
References
Bergsbaken, T., Fink, S. L., and Cookson, B. T. (2009). Pyroptosis: Host cell death and inflammation. Nat Rev Microbiol 7, 99–109.
Chimal-Monroy, J., Abarca-Buis, R. F., Cuervo, R., Diaz-Hernandez, M., Bustamante, M., Rios-Flores, J. A., Romero-Suarez, S., and Farrera-Hernandez, A. (2011). Molecular control of cell differentiation and programmed cell death during digit development. IUBMB Life 63, 922–29.
Cho, Y. S., Challa, S., Moquin, D., Genga, R., Ray, T. D., Guildford, M., and Chan, F. K. (2009). Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–23.
Dekkers, M. P., and Barde, Y. A. (2013). Developmental biology. Programmed cell death in neuronal development. Science 340, 39–41.
Denecker, G., Vercammen, D., Declercq, W., and Vandenabeele, P. (2001). Apoptotic and necrotic cell death induced by death domain receptors. Cell Mol Life Sci 58, 356–70.
Dexter, R. M., Wylie, A. H., and Raff, M. C. (1995). The role of apoptosis in development, tissue homeostasis and malignancy. Netherlands: Springer.
Elmore, S. (2007). Apoptosis: A review of programmed cell death. Toxicol Pathol 35, 495–516.
Elmore, S. A. (2015). Editorial. Semin Cell Dev Biol 39, 1–2.
Elmore, S. A., Boyle, M. C., Boyle, M. H., Cora, M. C., Crabbs, T. A., Cummings, C. A., Gruebbel, M. M., Johnson, C. L., Malarkey, D. E., McInnes, E. F., Nolte, T., Shackelford, C. C., and Ward, J. M. (2014). Proceedings of the 2013 National toxicology program satellite symposium. Toxicol Pathol 42, 12–44.
Ettcheto, M., Junyent, F., de Lemos, L., Pallas, M., Folch, J., Beas-Zarate, C., Verdaguer, E., Gomez-Sintes, R., Lucas, J. J., Auladell, C., and Camins, A. (2015). Mice lacking functional fas death receptors are protected from kainic acid-induced apoptosis in the Hippocampus. Mol Neurobiol 52, 120–29.
Fuchs, Y., and Steller, H. (2011). Programmed cell death in animal development and disease. Cell 147, 742–58.
Gavrieli, Y., Sherman, Y., and Ben-Sasson, S. A. (1992). Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119, 493–501.
Geske, F. J., Lieberman, R., Strange, R., and Gerschenson, L. E. (2001). Early stages of p53-induced apoptosis are reversible. Cell Death Differ 8, 182–91.
Gilmore, A. P. (2005). Anoikis. Cell Death Differ 12, 1473–77.
Giordano, G., Klintworth, H. M., Kavanagh, T. J., and Costa, L. G. (2008). Apoptosis induced by domoic acid in mouse cerebellar granule neurons involves activation of p38 and JNK MAP kinases. Neurochem Int 52, 1100–5.
Giovannetti, A., Pierdominici, M., Di Iorio, A., Cianci, R., Murdaca, G., Puppo, F., Pandolfi, F., and Paganelli, R. (2008). Apoptosis in the homeostasis of the immune system and in human immune mediated diseases. Curr Pharm Des 14, 253–68.
Glassford, A., Lee, J. E., Xu, L., and Giffard, R. G. (2002). Caspase inhibitors reduce the apoptotic but not necrotic component of kainate injury in primary murine cortical neuronal cultures. Neurol Res 24, 796–800.
Hall, P. A., Coates, P. J., Ansari, B., and Hopwood, D. (1994). Regulation of cell number in the mammalian gastrointestinal tract: The importance of apoptosis. J Cell Sci 107, 3569–77.
Han, J., Zhong, C. Q., and Zhang, D. W. (2011). Programmed necrosis: Backup to and competitor with apoptosis in the immune system. Nat Immunol 12, 1143–49.
He, S., Wang, L., Miao, L., Wang, T., Du, F., Zhao, L., and Wang, X. (2009). Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137, 1100–1111.
Holler, N., Zaru, R., Micheau, O., Thome, M., Attinger, A., Valitutti, S., Bodmer, J. L., Schneider, P., Seed, B., and Tschopp, J. (2000). Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1, 489–95.
Howden, R., Hanlon, P. R., Petranka, J. G., Kleeberger, S., Bucher, J., Dunnick, J., Nyska, A., and Murphy, E. (2005). Ephedrine plus caffeine causes agedependent cardiovascular responses in Fischer 344 rats. Am J Physiol Heart Circ Physiol 288, H2219–24.
Iguchi, K., Hirano, K., and Ishida, R. (2002). Activation of caspase-3, proteolytic cleavage of DFF and no oligonucleosomal DNA fragmentation in apoptotic Molt-4 cells. J Biochem 131, 469–75.
Kaczmarek, A., Vandenabeele, P., and Krysko, D. V. (2013). Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity 38, 209–23.
Kaufmann, S. H., Lee, S. H., Meng, X. W., Loegering, D. A., Kottke, T. J., Henzing, A. J., Ruchaud, S., Samejima, K., and Earnshaw, W. C. (2008). Apoptosis-associated caspase activation assays. Methods 44, 262–72.
Kerr, J. F., Wyllie, A. H., and Currie, A. R. (1972). Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26, 239–57.
Krajewska, M., You, Z., Rong, J., Kress, C., Huang, X., Yang, J., Kyoda, T., Leyva, R., Banares, S., Hu, Y., Sze, C. H.,Whalen, M. J., Salmena, L., Hakem, R., Head, B. P., Reed, J. C., and Krajewski, S. (2011). Neuronal deletion of caspase 8 protects against brain injury in mouse models of controlled cortical impact and kainic acid-induced excitotoxicity. PLoS One 6, e24341.
Kroemer, G., El-Deiry, W. S., Golstein, P., Peter, M. E., Vaux, D., Vandenabeele, P., Zhivotovsky, B., Blagosklonny, M. V., Malorni, W., Knight, R. A., Piacentini, M., Nagata, S., and Melino, G. (2005). Classification of cell death: recommendations of the nomenclature committee on cell death. Cell Death Differ 12, 1463–67.
Kroemer, G., Galluzzi, L., Vandenabeele, P., Abrams, J., Alnemri, E. S., Baehrecke, E. H., Blagosklonny, M. V., El-Deiry, W. S., Golstein, P., Green, D. R., Hengartner, M., Knight, R. A., Kumar, S., Lipton, S. A., Malorni, W., Nunez, G., Peter, M. E., Tschopp, J., Yuan, J., Piacentini, M., Zhivotovsky, B., and Melino, G. (2009). Classification of cell death: Recommendations of the nomenclature committee on cell death 2009. Cell Death Differ 16, 3–11.
Krysko, D. V., Vanden Berghe, T., Parthoens, E., D’Herde, K., and Vandenabeele, P. (2008). Methods for distinguishing apoptotic from necrotic cells and measuring their clearance. Methods Enzymol 442, 307–41.
Kung, G., Konstantinidis, K., and Kitsis, R. N. (2011). Programmed necrosis, not apoptosis, in the heart. Circ Res 108, 1017–36.
Lang, F., Lang, E., and Foller, M. (2012). Physiology and pathophysiology of eryptosis. Transfus Med Hemother 39, 308–14.
Leist, M., Volbracht, C., Kuhnle, S., Fava, E., Ferrando-May, E., and Nicotera, P. (1997). Caspase-mediated apoptosis in neuronal excitotoxicity triggered by nitric oxide. Mol Med 3, 750–64.
Levin, S., Bucci, T. J., Cohen, S. M., Fix, A. S., Hardisty, J. F., LeGrand, E. K., Maronpot, R. R., and Trump, B. F. (1999). The nomenclature of cell death: Recommendations of an ad hoc committee of the society of toxicologic pathologists. Toxicol Pathol 27, 484–90.
Lieberthal, W., and Levine, J. S. (1996). Mechanisms of apoptosis and its potential role in renal tubular epithelial cell injury. Am J Physiol 271, F477–88.
Linkermann, A., and Green, D. R. (2014). Necroptosis.N Engl J Med 370, 455–65.
Loo, D. T. (2011). In situ detection of apoptosis by the TUNEL assay: An overview of techniques. Methods Mol Biol 682, 3–13. Accessed December 29, 2015. http://lifescience. roche.com/wcsstore/RASCatalogAssetStore/ Articles/BIOCHEMICA_98_3_p36-41.pdf.
Lu, L., and Osmond, D. G. (2000). Apoptosis and its modulation during B lymphopoiesis in mouse bone marrow. Immunol Rev 175, 158–74.
Martin, L. J., Al-Abdulla, N. A., Brambrink, A. M., Kirsch, J. R., Sieber, F. E., and Portera-Cailliau, C. (1998). Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res Bull 46, 281–309.
Meier, P., Finch, A., and Evan, G. (2000). Apoptosis in development. Nature 407, 796–801.
Morgan, D. L., Little, P. B., Herr, D. W., Moser, V. C., Collins, B., Herbert, R., Johnson, G. A., Maronpot, R. R., Harry, G. J., and Sills, R. C. (2004). Neurotoxicity of carbonyl sulfide in F344 rats following inhalation exposure for up to 12 weeks. Toxicol Appl Pharmacol 200, 131–45.
Newton, K. (2015). RIPK1 and RIPK3: Critical regulators of inflammation and cell death. Trends Cell Biol 25, 347–53.
Nyska, A., Murphy, E., Foley, J. F., Collins, B. J., Petranka, J., Howden, R., Hanlon, P., and Dunnick, J. K. (2005). Acute hemorrhagic myocardial necrosis and sudden death of rats exposed to a combination of ephedrine and caffeine. Toxicol Sci 83, 388–96.
Padanilam, B. J. (2003). Cell death induced by acute renal injury: A perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 284, F608–27.
Proskuryakov, S. Y., Konoplyannikov, A. G., and Gabai, V. L. (2003). Necrosis: A specific form of programmed cell death? Exp Cell Res 283, 1–16.
Raff, M. C., Barres, B. A., Burne, J. F., Coles, H. S., Ishizaki, Y., and Jacobson, M. D. (1993). Programmed cell death and the control of cell survival: Lessons from the nervous system. Science 262, 695–700.
Rizzo, L. V., Secord, E. A., Tsiagbe, V. K., Umetsu, D. T., Dekruyff, R. H., Simmons, W. J., and Thorbecke, G. J. (1998). Components essential for the generation of germinal centers. Dev Immunol 6, 325–30.
Ryter, S. W., Mizumura, K., and Choi, A. M. (2014). The impact of autophagy on cell death modalities. Int J Cell Biol 2014, 502676.
Savolainen, S. M., Foley, J. F., and Elmore, S. A. (2009). Histology atlas of the developing mouse heart with emphasis on E11.5 to E18.5. Toxicol Pathol 37, 395–414.
Shen, H., Kreisel, D., and Goldstein, D. R. (2013). Processes of sterile inflammation. J Immunol 191, 2857–63. Slee, E. A., Adrain, C., and Martin, S. J. (1999). Serial killers: Ordering caspase activation events in apoptosis. Cell Death Differ 6, 1067–74.
Taylor, R. C., Cullen, S. P., and Martin, S. J. (2008). Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9, 231–41. Vandenabeele, P., Galluzzi, L., Vanden Berghe, T., and Kroemer, G. (2010). Molecular mechanisms of necroptosis: An ordered cellular explosion. Nat Rev Mol Cell Biol 11, 700–14.
Vanden Berghe, T., Vanlangenakker, N., Parthoens, E., Deckers, W., Devos, M., Festjens, N., Guerin, C. J., Brunk, U. T., Declercq, W., and Vandenabeele, P. (2010). Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ 17, 922–30.
Wang, Q., Yu, S., Simonyi, A., Sun, G. Y., and Sun, A. Y. (2005). Kainic acidmediated excitotoxicity as a model for neurodegeneration. Mol Neurobiol 31, 3–16.
White, M. K., and Cinti, C. (2004). A morphologic approach to detect apoptosis based on electron microscopy. Methods Mol Biol 285, 105–11.
Wijsman, J. H., Jonker, R. R., Keijzer, R., van de Velde, C. J., Cornelisse, C. J., and van Dierendonck, J. H. (1993). A new method to detect apoptosis in paraffin sections: In situ end-labeling of fragmented DNA. J Histochem Cytochem 41, 7–12.
Yamaguchi, K., Uzzo, R., Dulin, N., Finke, J. H., and Kolenko, V. (2004). Renal carcinoma cells undergo apoptosis without oligonucleosomal DNA fragmentation. Biochem Biophys Res Commun 318, 710–13.
Yuste, V. J., Bayascas, J. R., Llecha, N., Sanchez-Lopez, I., Boix, J., and Comella, J. X. (2001). The absence of oligonucleosomal DNA fragmentation during apoptosis of IMR-5 neuroblastoma cells: Disappearance of the caspase-activated DNase. J Biol Chem 276, 22323–31.
Zakharyan, R. A., and Pogosyan, R. G. (1978). Glucocorticoid induction of the degradation of lymphocyte chromatin DNA into regularly repeating fragments in vivo. Doklady Akademii Nauk Armyanskoi SSR 67 (2): 110–114. ISSN 0366-8606.
Zeiss, C. J. (2003). The apoptosis-necrosis continuum: Insights from genetically altered mice. Vet Pathol 40, 481–95.
Zhang, D. W., Shao, J., Lin, J., Zhang, N., Lu, B. J., Lin, S. C., Dong, M. Q., and Han, J. (2009). RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–36.
Zhang, X. M., and Zhu, J. (2011). Kainic acid-induced neurotoxicity: Targeting glial responses and glia-derived cytokines. Curr Neuropharmacol 9, 388–98.
Ziegler, U., and Groscurth, P. (2004). Morphological features of cell death. News Physiol Sci 19, 124–28.