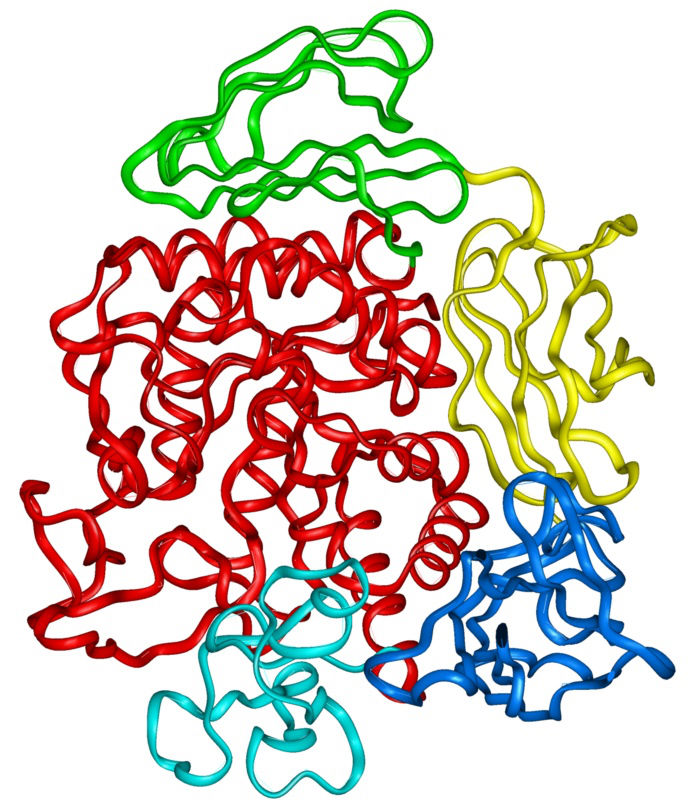
Genetic and Rat Toxicity Studies of Cyclodextrin Glucanotransferase
Robert Maronpot2017-01-11T22:13:22+00:00Highlights • Bacterial cyclodextrin glucanotransferase (CGTase) is used to produce a water soluble form of glycosylated isoquercitrin. • Genotoxicity battery on CGTase and sodium sulfate negative for mutations and DNA damage. • No evidence of systemic toxicity in 90-day rat toxicity study of CGTase Introduction Microbiologically derived cyclodextrin glucanotransferase (CGTase) is used commercially as a processing [...]